Angiogenesis – otherwise known as neovascularization – is when new blood vessels are generated from pre-existing vasculature. Angiogenesis is an indication of cancer, playing a key role in enabling the growth, progression, and metastasis of tumor growth. This article discusses the differences between normal angiogenesis physiology, and tumor vascularization, also highlighting therapeutic targets aimed at suppressing angiogenesis. Today, researchers favor a combination of strategies to target different stages of angiogenesis, with an aim to prevent the growth and expansion of the tumor.
Angiogenesis
Angiogenesis involves the generation of new blood vessels from pre-existing vasculature. It is a normal process in the body’s growth and development and is vital in the formation of arteries, veins, and capillaries in the embryo. In adults, the proliferation of new blood vessels takes place, and this process is essential in the repair and regeneration of tissue at a time of wound healing.
In normal tissues, angiogenesis is a carefully regulated process that is coordinated by pro- and antiangiogenic factors like vascular endothelial growth factor (VEGF) and endostatin respectively. As a result, it produces well-structured uniform vasculature (Figure 1).
Angiogenesis also plays a key role in cancer by enabling tumor growth, progression, and metastasis. During the development of a tumor, the availability of oxygen, glucose and other necessary nutrients from the existing vasculature limits its size.
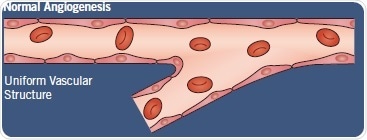
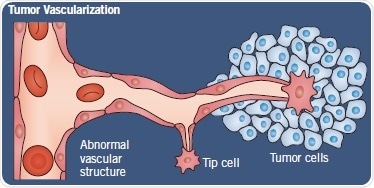
Fig 2. Normal angiogenesis versus tumor vascularization. Image credit: Tocris Bioscience
Tumors are heterogeneous in nature as a consequence of their genetic instability. Thus, tumor angiogenesis can differ significantly from physiological angiogenesis. As a result, this produces poorly formed blood vessels with aberrant blood flow and differing permeability. Moreover, factors such as a tumor’s p53 status can affect blood vessel formation because p53 regulates angiogenic cytokines (Figure 2).
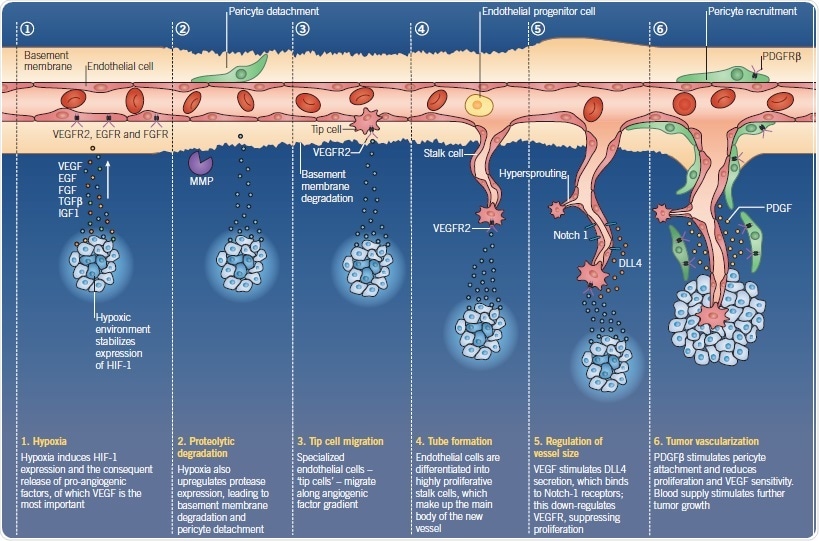
Fig 1. The process of angiogenesis. Image credit: Tocris Bioscience
Tumor Vascularization – Therapeutic Targets
Hypoxia is a primary trigger in the growth of new blood vessels in a tumor. Hypoxia-inducible factor 1 (HIF-1) undergoes prolyl hydroxylation, which thereby facilitates ubiquitination and its destruction. The expression of HIF-1 is stabilized in a hypoxic environment. During this, HIF-1α associates with HIF-1β, thus initiating transcription by binding to the response element of HIF-responsive genes, as well as binding the cofactors p300/CBP and pyruvate kinase isoform M2 (PKM2).
This results in the secretion of proangiogenic factors that encourage the formation of new vessels. Tumors secrete a number of proangiogenic factors, inducing endothelial cell proliferation and facilitating vessel patterning. Studying their receptors is vital in antiangiogenic research; key targets include VEGF receptor 2 (VEGFR2), epidermal growth factor receptor (EGFR), and fibroblast growth factor receptor (FGFR). VEGF is the most important and commonly secreted proangiogenic factor, which binds VEGFR2 and neuropilin. As a result, this increases vasodilation and vascular permeability.
Tumor cells and VEGF-stimulated endothelial cells also secrete matrix metalloproteases (MMPs). Such a scenario helps in the breakdown of the extracellular matrix (ECM) and mobilization of proangiogenic proteins from the stroma. Further, activation of the platelet-derived growth factor receptor β (PDGFRβ) stimulates the attachment of pericytes along the new vessel branch, thereby forming cell-to-cell and gap junctions. This is followed by basement membrane formation.
In fact, pericyte attachment reduces the proliferation of endothelial cells and their sensitivity to VEGF.
Finally, what plays a key role in the differentiation and shaping the new vascular branch is Notch signaling. VEGF stimulates the secretion of Delta-like 4 (DLL4) in the tip cell, binding to Notch-1 receptors expressed on the stalk cells.
This results in down-regulation of VEGFR, which suppresses endothelial cell proliferation, thus regulating the size of the vessel.
Each of these stages presents an opportunity for therapeutic interventions. Vascularization of the tumor can be prevented by interrupting one or more of these stages, thus cutting off vital nutrient supplies and oxygen, as well as preventing waste removal.
The growth of the tumor can also be stunted by attenuating tumor vascularization. This can also cause the tumor environment to become so toxic that the tumor cells die. The stabilization of angiogenesis in tumors is another strategy, which facilitates the delivery of cytotoxic drugs to the tumor cells.
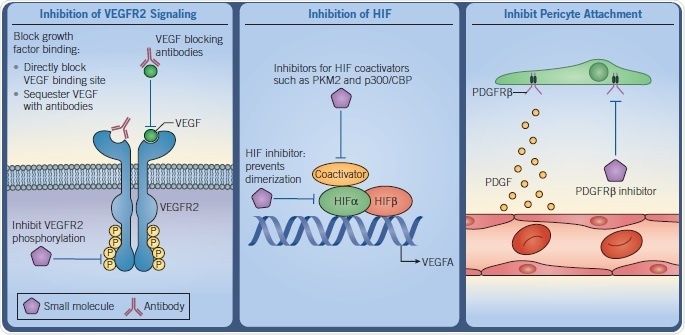
Fig 4. Diagrams demonstrating the inhibition of VEGFR2 Signaling, HIF and Pericyte attachment. Image credit: Tocris Bioscience
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.