Alzheimer’s disease (AD) is a degenerative brain disorder that is, in fact, the most common form of dementia. AD is characterized by a decline in faculties such as memory, language, problem-solving and other cognitive skills that affect a person’s ability to perform everyday tasks.
This fall occurs due to the loss of neurons (in particular, pyramidal cells) in those regions of the brain which are involved in cognitive function.
Over time, the disease eventually affects and impairs areas of the brain that enable a person to carry out basic functions – such as walking and swallowing. Often, people in the final stages of Alzheimer’s are bed-ridden and require intense, round-the-clock care.
Normal Memory Processing
In a healthy brain, memory is the function that encodes, stores, and later retrieves knowledge of the world. Memory can be categorized into short-term and long-term memory. As the name implies, short-term (or working memory) allows recall for a period between several seconds to a minute without rehearsal. By nature, short-term memory has a limited capacity.
In contrast, long-term memory can store much more information for periods as long as an entire lifetime. Long-term memory can be sub-categorized into explicit, or conscious, and implicit i.e. unconscious memory. Explicit memory can further be split into semantic memory, which comprises facts and general knowledge, and episodic memory – i.e. personally experienced events. Implicit memory, in turn, comprises classical conditioning, priming (or the enhanced identification of objects or words) and procedural memory – which involves cognitive and motor skills memory (Figure 1).
Semantic memory is controlled by the region of the brain responsible for that particular semantic task. For instance, the concept of tools comes from the motor cortex. In comparison, episodic memory is mediated by two areas – the entorhinal cortex and hippocampus (Figure 2).
In the course of AD, both episodic and semantic memory are affected very early on. As with the progress of the disease, these deficits progressively become worse and are accompanied by impairments in implicit or unconscious conceptual memory. However, the implicit perceptual memory is not affected and seems to be preserved.
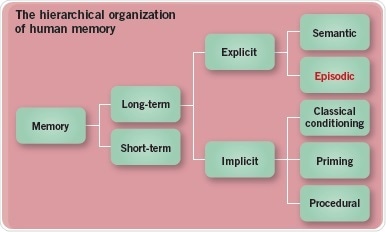
Fig. 1: The hierarchical organization of human memory. Image credit: Tocris Bioscience
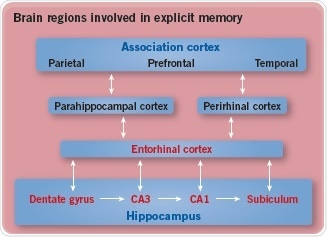
Fig. 2: Brain regions involved in explicit memory. Image credit: Tocris Bioscience
Alzheimer’s Disease Neuropathology
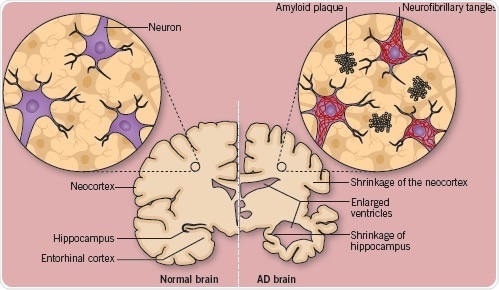
Fig 3. A comparison of a normal, healthy brain, with a brain affected with advanced Alzheimer's disease. Image credit: Tocris Bioscience
Figure 3 depicts a normal, healthy brain alongside a brain with advanced AD. As demonstrated, the AD brain displays severely enlarged ventricles, shrinkage of the neocortex and extreme shrinkage of the hippocampus (a region that plays a critical role in memory). This level of atrophy reflects the loss of vulnerable neurons – especially the pyramidal, cholinergic, noradrenergic, and serotonergic neurons.
One of the main hallmarks of AD is the formation of dystrophic neurites around a central core of amyloid (plaques). AD also involves the formation of abnormal filaments (i.e. neurofibrillary tangles), which are made up of a highly phosphorylated form of the microtubule-associated protein tau in the perikaryia of certain neurons, accompanied by neuropil threads in axons and nerve terminals.
Progression of Alzheimer’s Disease
At the present time, diagnosing AD definitively can only be done through a postmortem examination of the brain. In fact, the disease takes root about 15 years before symptoms manifest and start to emerge in a prodromal form i.e. through amnesic mild cognitive impairment. AD then progresses through six stages, depending on neurofibrillary tangles in distinct brain regions (Figure 4).
The first to be affected is the entorhinal cortex, which leads to the episodic memory being impaired. Once the disease spreads to other areas of the neocortex, it leads to impairment in additional domains of cognitive function, finally manifesting the syndrome of dementia becomes evident.
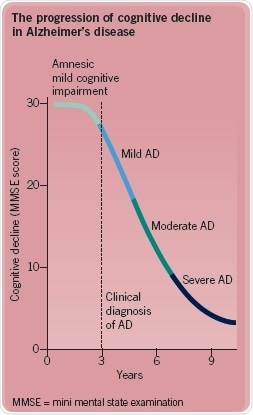
Fig. 4: The progression of cognitive decline in Alzheimer's disease. Image credit: Tocris Bioscience
Braak and Braak have compartmentalized AD’s progression into six stages (I to VI) on the basis of the pattern and severity of Aβ deposition and neurofibrillary changes. Aβ deposition is depicted in the brains on the left in Figure 5 while neurofibrillary changes can be seen in the brains on the right, which are indicated as mild (stages I to II), moderate (stages III to IV), and severe (stages V to VI) (Figure 5). The figure below is based on Braak et al. (1998), Palmer (2002) and Palmer (2011).
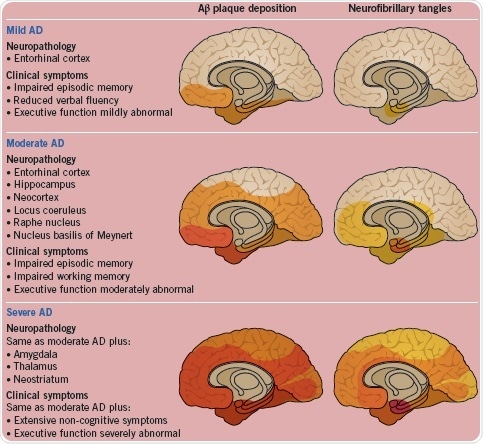
Fig. 5: The neuropathology and clinical symptoms shown as Alzheimer's progresses. Image credit: Tocris Bioscience
The disease starts within excitatory pyramidal neurons in the entorhinal cortex. Subsequently, it spreads from the hippocampus and other limbic structures, thus affecting pyramidal neurons in the neocortex and ascending cholinergic, noradrenergic, and serotonergic neurons.
An early change in the disease is the degeneration of pathways that link the entorhinal cortex and the hippocampus. In the mild/moderate stage, projections from the nucleus basalis of Meynert, the locus coeruleus, and the raphe nucleus are lost.
The final stages of AD involve the loss of GABAergic interneurons.
AD Drug Targets
AD drug discovery’s major goal is a compound that slows or stops the progression of neurodegenerative change that characterizes this disease. In addition to the enzyme targets displayed in Figure 6, anti-Aβ monoclonal antibodies are a major focus. These efforts are of vital understanding in the pathogenesis of AD.
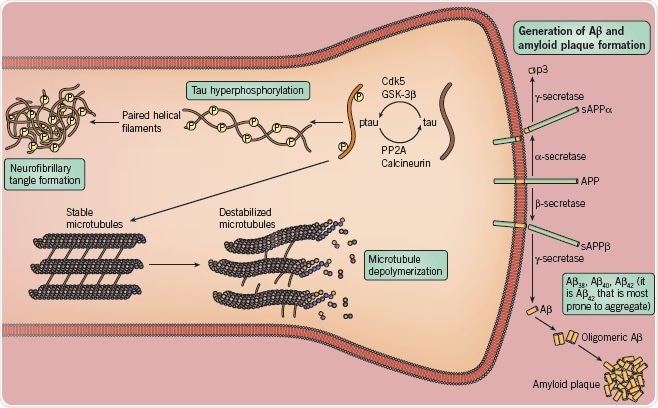
Fig 6. Enzyme targets to be the focus of AD drug targets. Image credit: Tocris Bioscience
A major breakthrough was the discovery that deposits of Aβ – a peptide sub-fragment of APP – are present in both diffuse and neuritic plaques. Additionally, it was also revealed that mutations in both APP and enzymes involved in its metabolism cause a majority of cases of familial AD. This resulted in the formation of the amyloid hypothesis – which states that deposits of Aβ peptide are responsible for the pathophysiological changes associated with AD.
Aβ peptides are produced through the action of two cleaving enzymes (β- and γ-secretase) on APP, with the resulting peptide being 38, 40 or 42 amino acids in length. The largest form of these (Aβ42) appears to be critical, through its accumulation and oligomerization.
The main component of NFTs is Tau, which has become an increasingly popular alternative to Aβ in AD drug discovery and is being used as a viable target. Hyperphosphorylated tau interacts with other tau threads to form NFTs inside neurons, in particular, pyramidal cells. Hyperphosphorylated tau is also responsible for destabilizing microtubules – which are integral in the maintenance of the shape and function of neurons.
Thus far, drug discovery efforts have focused on inhibitors of tau phosphorylation and tau aggregation, in addition to compounds that promote NFT disassembly.
Abbreviations: Aβ – amyloid beta; APP – amyloid precursor protein; Cdk5 – cyclin-dependent kinase 5; GSK-3β – glycogen synthase kinase 3β; NFT – neurofibrillary tangles; PP2A – protein phosphatase 2A.
References
- Braak et al (1998) , J. Neural Transm. Suppl. 54 97
- Palmer et al (2002) , Trends Pharmacol. Sci. 23 426
- Palmer et al (2011) , Trends Pharmacol. Sci. 32 141
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.