Autoimmune diseases, including rheumatoid arthritis (RA), occur as a consequence of a breakdown in immune tolerance, for unknown reasons. RA impacts nearly 1% of the global population, representing a lifetime risk of 2% for men and 4% for women.
Although certain successful immunotherapies, including anti-CD20, anti-TNF, and CTLA-4Ig, have made a huge contribution to the treatment of RA in recent years, several patients' response to therapy continues to be insufficient. Furthermore, these expensive therapies are not curative, meaning treatment needs to be maintained for many years in order to keep the disease under control.
While the etiology and pathogenesis of RA still elude researchers, investigations have detected a genetic predisposition, with variants of multiple genes, such as human leukocyte antigen DRB1 (HLA-DRB1), CTLA-4, PTPN22, CD28, and IRF5, possessing a robust link with the condition.
In spite of this considerable genetic predisposition, the coincidence of RA among monozygotic twins is not high (12–20%), indicating the importance of environmental and epigenetic factors in disease development(1). The environmental factor most commonly linked to RA is cigarette smoking, with alcohol consumption apparently constituting a moderate protective effect.
RA Pathology
RA is a chronic destructive inflammatory disease that is distinguished by acute swelling, stiffness, and pain of the joints. Among healthy individuals, the synovial membrane, with which the joint capsule is lined, functions as a lubricant of the joint by generating synovial fluid.
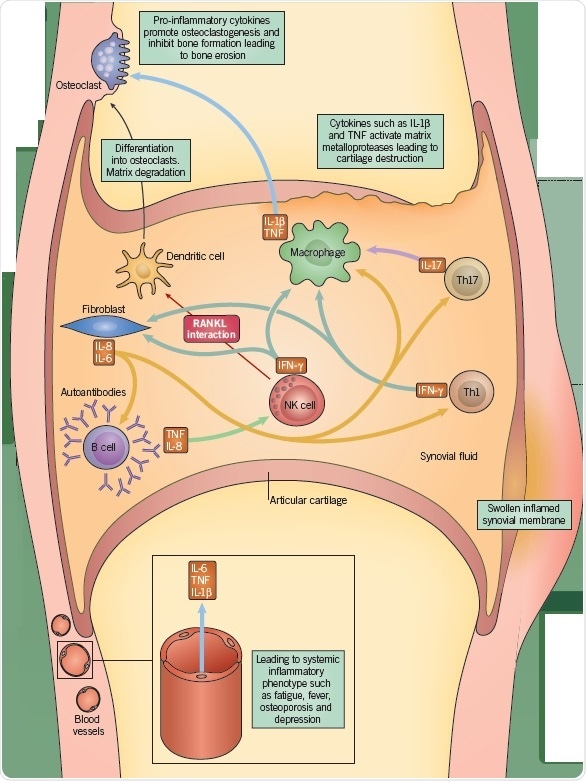
The synovial membrane comprises two principle immune cell types, macrophages and synovial fibroblasts (synoviocytes), which occur within the connective tissue. However, in RA, there is an incursion of B cells, NK cells, and T cells, as well as additional chronically inflamed cell types. The synoviocytes undergo activation and permeate the surrounding cartilage, leading to cartilage degradation and bone erosion.
RA Inflammation
Antigen-activated T cells generate pro-inflammatory cytokines, such as IL-17, which stimulate macrophages, monocytes, and synovial fibroblasts into producing additional pro-inflammatory cytokines, IL-1, IL-6, and TNF-α. This causes the recruitment of further immune cells, such as B cells and NK cells, which consequently secrete, respectively, immunoglobulins, such as rheumatoid factor, and IFN-γ. These combined proinflammatory cytokines make an overall contribution to the articular inflammation. Moreover, the stimulation of fibroblasts, lymphocytes, and macrophages can cause angiogenesis, which might account for the heightened vascularity observed in the synovium of RA patients.
Furthermore, the endothelial cells in these newly constituted vessels express high levels of adhesion markers, causing multiplied inflammatory cell recruitment and infiltration. Activated CD4+ T cells express osteoprotegerin ligands, which stimulate osteoclastogenesis, while NK cells from the RA synovium similarly express increased RANKL, further stimulating osteoclastogenesis. This leads to bone erosion.
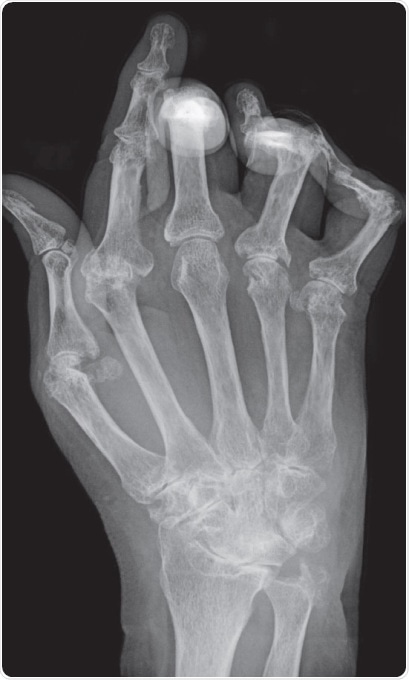
Figure 1. Typical X-ray of a hand with advanced rheumatoid arthritis. Bone erosion, cartilage degradation, and bone displacement are classic features of this disease (from Bernd Brägelmann via Wikimedia Commons)
Epigenetic and Environmental Evidence in RA
The low concordance rate of RA between monozygotic twins and the late onset of RA represent evidence that epigenetic alterations may be critical for disease development. A variety of possible environmental risk factors have been suggested. Periodontitis is one such factor.
It has been demonstrated that Porphyromonas gingivalis, a cause of periodontitis, expresses an enzyme manifesting peptidylarginine deiminase (PAD) activity (referred to as PPAD in P. gingivalis), which stimulates the citrullination of peptide residues.
Autoantibodies against citrullinated peptides occur in the majority of RA patients. Indeed, it has been indicated that PPAD activity might precipitate the development of autoantibodies in RA(2).
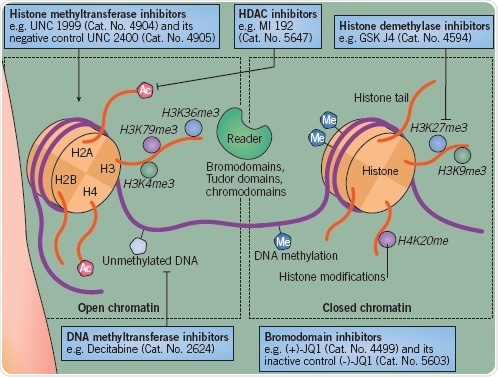
Figure 2.
Cigarette smoking represents an additional critical risk factor. This is also linked to periodontitis, and can increase RA intensity. The reduction of histone deacetylase (HDAC) SIRT1 and SIRT2 expression in macrophages within the lungs is one mechanism implicated in cigarette smoking(3).
Epigenetic Modifications in RA
Several epigenetic alterations have been determined to embody pathogenic importance in RA. Synovial fibroblasts in RA exhibit a DNA methylome pattern distinct from healthy fibroblasts. Likewise, CD4+ T cell hypomethylation in RA patients has been demonstrated to cause dysregulated cell function.
In Tregs, DNA methylation of promoter and enhancers of key genes can also restrict the capacity of these cells to prevent the ongoing inflammation(4). RA patients exhibit a substantially multiplied expression of HDAC activity in PBMCs, indicating that this pathway has undergone dysregulation, while research into synovial fibroblasts has also indicated a growth in HDAC activity(5).
Studies regarding histone methylation in the context of RA are uncommon, even though the histone methyltransferase EZH2 has been indicated to be over-expressed in RA fibroblasts. This suggests that H3K27me3 might undergo an alteration in RA.
Epigenetic Pathways for Potential Intervention in RA
The primary objective for RA drug discovery is to determine a compound that inhibits the inflammatory cascade within the joint and causes a reversal of the damage connected to RA. Although contemporary medicines are accomplished in the prevention of inflammation, they are not curative and are needed for the lifetime of the patient.
One possible benefit of focusing on an epigenetic pathway in RA is the facility to induce longer-term remission. Research in the 1990s utilizing the DNA methylation inhibitor 5-azacytidine revealed that targeting this pathway was not likely to be beneficial as the drug generated self-reactive CD4+ T cells and a lupus-like disease to be induced.
Nevertheless, HDAC inhibitors can contribute to the reduction of multiple pro-inflammatory cytokines. One such compound, givinostat, has been determined in clinical trials to treat juvenile idiopathic arthritis and has been given Orphan Drug designation for this indication in the European Union.
More recently, it has been shown that targeting further epigenetic pathways might be of benefit for RA. For example, BET and CBP/p300 bromodomain inhibition reduce the generation of IL-8/IL-6 from fibroblasts and IL-17A from Th17 T cells(6). Likewise, inhibition of the JMJD3/UTX jumonji histone demethylases can cause a reduction of the expression of IL-17A in Th17 cells(7). At present, drug discovery endeavors are focusing on the identification of compounds that can target other epigenetic readers, writers, and erasers of the histone code, such as lysine demethylases, kinases, and histone methyltransferases.
References and Further Reading
- Svendsen et al. (2013) PloS one 8(2):e57304.
- Wegner et al. (2010) Arthritis Rheum. 62(9):2662.
- Yang et al. (2007) Am.J.Physiol.Lung Cell.Mol.Physiol. 292(2):L567.
- Cribbs et al. (2014) Arthritis Rheum. 66(9):2344.
- Cribbs et al. (2015) Ther.Adv. Musculoskelet.Dis. 7(5):206.
- Klein et al. (2016) Ann.Rheum.Dis. 75(2):422.
- Oppermann (2013) Arthritis Res.Ther. 15(2):209.
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.