Otto Warburg was the first to discover in 1924 that cancer cells generated a large proportion of their adenosine triphosphate (ATP) by metabolizing glucose via aerobic glycolysis. This was in sharp contrast to ATP production through oxidative phosphorylation (OXPHOS) in normal cells.
Initially, it was believed that this Warburg effect caused cancer, but it was later discovered that this shift to glycolytic metabolism was an effect of the transformation of cancer cells. The regulation of cellular metabolic pathways in cancer cells can be altered by genetic changes and epigenetic modifications. These distinct metabolic circuits could provide viable targets in cancer therapy.
Abnormal Cancer Metabolism
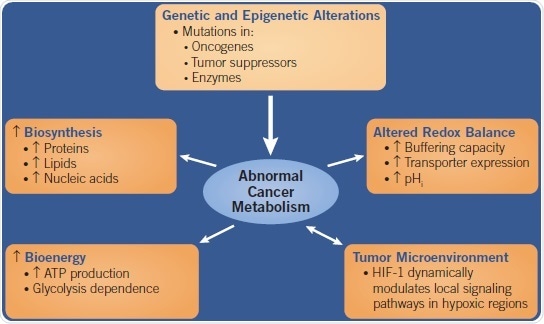
Fig 1. A flow chart showing the causes and effects of Abnormal Cancer Metabolism. Image credit: Tocris Bioscience
Main Targets in Cancer Metabolism
Glycolysis, Pentose Phosphate Pathway, and Transporters
Enhanced rates of glycolysis (approximately 200-fold) are known to produce increased levels of ATP in a far more rapid manner than OXPHOS. However, this process is far less efficient, resulting in an increased demand for glucose. As such, glucose transporter (GLUT) expression is frequently increased in cancer cells, along with monocarboxylate transporter (MCT) expression. Both remove the increasing levels of lactate from the cells.
Another adaptation that is commonly seen is an increased number of glutamine transporters. The first step in glutamine catabolism involves the hydrolysis of glutamine into glutamate and ammonia by glutaminase (GLS1), which is vital in lipid biosynthesis and NADPH production.
Further, this also causes an increase in the flux through the pentose phosphate pathway (PPP). The PPP is required for the generation of precursors for amino acid synthesis and NADPH (an integral component in lipid and nucleotide synthesis, as well as redox homeostasis). Glucose is directed into either the PPP or glycolysis pathway (or both), depending on the requirements of the cancer cell.
For instance, during high oxidative stress, cancer cells divert the flux of glucose away from glycolysis into the PPP to produce more NADPH.
Krebs Cycle
Glucose breaks down into pyruvate, which then gets transported into the mitochondria, where it is converted into acetyl-CoA. This then enters the Krebs cycle. The process produces energy in the form of ATP, as well as precursors for amino acid synthesis and the reducing agent NADPH.
Glutamate dehydrogenase (GDH) is one of the major enzymes that feeds into the Krebs cycle, converting glutamate to α-ketoglutarate (α-KG). α-KG is an essential component in the Krebs cycle. Inhibition of GDH is known to suppress the use of glutamine in the Krebs cycle and sensitize glioblastoma cells to glucose withdrawal.
Mutant forms of isocitrate dehydrogenase (IDH), which have been linked to oncogenesis, use α-KG as a substrate. Mutant IDH converts α-KG to D-2-hydroxyglutarate (D2HG), which results in high intracellular levels of D2HG. Further, D2HG competitively blocks α-KG binding at a family of enzymes called 2-OG-dependent dioxygenases, which function as regulators of important epigenetic events. What’s more, IDH mutations impair cell redox capacity.
Lipidogenesis
Recent evidence has shown that the initiation of cell proliferation relies more on lipid metabolism than glycolysis, in certain types of cancer such as prostate cancer. In fact, the targeting of fatty acid synthesis can hamper a cell’s ability to proliferate and survive because it limits lipid membrane production. It also blocks β-oxidation of fatty acids in mitochondria.
pH and Redox Balance
Cancer cells can survive in their hostile microenvironments due to the increased expression of proton pumps and ion transporters. However, aberrant regulation of the hydrogen ions can cause a reversal of the pH gradient across tumor cell membranes.
This then results in a more basic intracellular pH (pHi) and a more acidic extracellular pH (pHe). It is critical to the survival of cancer cells that the intracellular environment does not become acidified since such a scenario could induce apoptosis.
Altered Regulation of Metabolic Pathways - Abnormal Cancer Metabolism in Cancer Cells
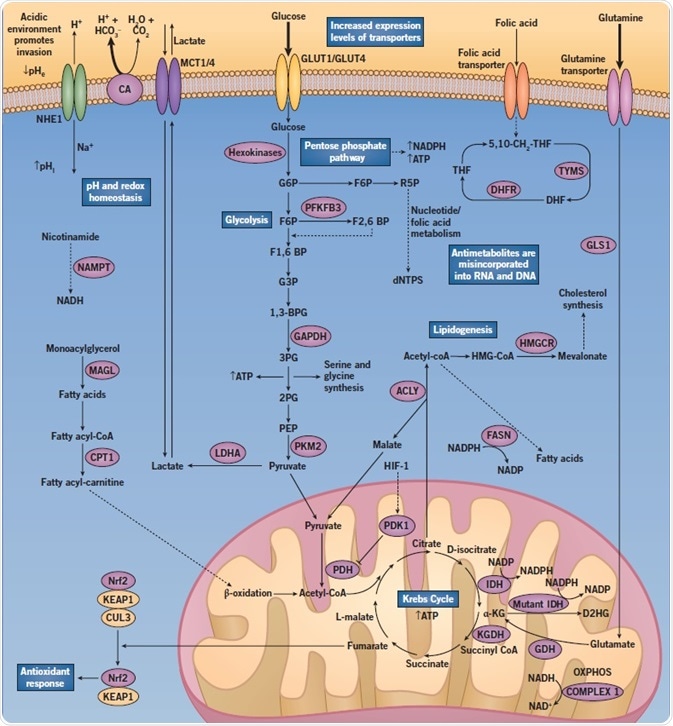
Fig 2. A diagram showing the altered regulation of metabolic pathways when abnormal cancer metabolism in cancer cells occurs. Image credit: Tocris Bioscience
References
- Butler et al (2013) Stalling the engine of resistance: targeting cancer metabolism to overcome therapeutic resistance. Cancer Res. 73 2709.
- Chartoumpekis et al (2015) Keap1/Nrf2 pathway in the frontiers of cancer and non-cancer cell metabolism. Biochem. Soc. Trans. 43 639.
- Doherty et al (2014) Blocking lactate export by inhibiting the Myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 74 908.
- Feng et al (2012) Dysregulated lipid metabolism in cancer. World J. Biol. Chem. 3 167. Galluzzi et al (2013) Metabolic targets for cancer therapy. Nat. Rev. Drug Discov. 12 829. Pavlova and Thompson (2016) The emerging hallmarks of cancer metabolism. Cell Metab. 23 27.
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.