Major depressive disorder (MDD) is often referred to as major depression. The condition is characterized by core symptoms that include a depressed mood and a loss of interest and/or pleasure.
MDD can manifest as other symptoms as well, including significant weight changes (either weight loss or gain), disturbances in the sleep pattern (insomnia or hypersomnia), fatigue, the diminished ability to think or concentrate, feelings of worthlessness or guilt, recurrent thoughts of death or suicide, and psychomotor agitation or retardation.
Further, in addition to producing clinically significant distress, major depressive episodes are almost always accompanied by some level of social and/or occupational impairment. This negatively impacts the quality of one’s life and contributes to the societal burden associated with loss of work and health care costs.
Today, approximately 15% of the population in developed countries has been diagnosed with MDD in their lifetime and around 5% has suffered a major depressive episode during the last year. In women, the risk of developing MDD is almost double. Unfortunately, the majority of people affected by MDD do not receive standard treatment.
Etiology of Depression
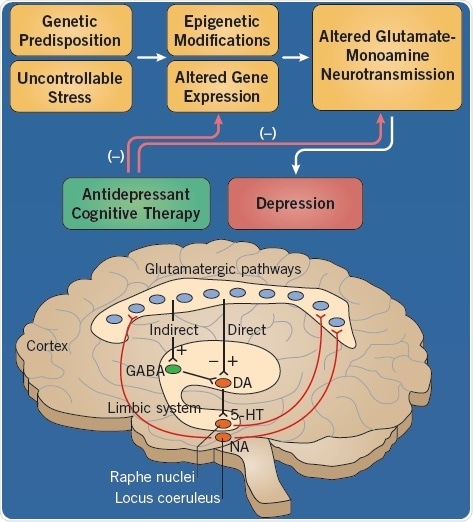
Fig 1. The etiology of depression. Image credit: Tocris Bioscience
Depression can be triggered by a combination of a number of etiological factors, each of which are capable of altering the functioning of the areas of the brain, such as the limbic system, which are associated with mood (Figure 1). There is a strong contribution of genetics in the condition of depression, and although several gene candidates have been reported, none have been irrefutably identified.
Gene expression may be modified by stressful life experiences, resulting in enduring changes in neurotransmission in limbic areas of the brain. The negative effects of these etiological factors can be limited by antidepressant drugs and cognitive therapy.
Pharmacology of Antidepressant Drugs
Today, approved antidepressants (ADs) are able to increase the synaptic availability of one or more of the biogenic amine transmitters – noradrenaline, dopamine, and serotonin. These ADs inhibit the monoamine oxidase (MAO), which is an enzyme responsible for the metabolism of biogenic amines.
Alternatively, they also inhibit one or more of the transport proteins that remove these biogenic amines from the synaptic cleft. The latter process is the principal way to terminate the actions of these neurotransmitters. The majority of ADs currently in use selectively block the uptake of serotonin and/or noradrenaline.
Well-regulated, double-blind clinical trials involve biogenic amine-based ADs which require ≥2 weeks of treatment before providing meaningful relief. This is considered a “therapeutic lag” that has been hypothesized to represent neuroplastic changes preceding a reduction in depressive symptomatology.
The objective behind the identification of novel targets bypassing the aminergic synapse is to develop drugs that are significantly more effective and quick to act, in comparison to currently approved agents. Over the past decade, clinical studies have been undertaken with NMDA receptor antagonists like ketamine and traxoprodil, and the muscarinic receptor antagonist scopolamine have demonstrated the possibility of producing a rapid and robust therapeutic response in depressed individuals.
In fact, many of these individuals do not show responses to biogenic amine based agents. While a multitude of promising targets have been identified, new therapeutics will have to have a high safety bar, considering the availability of alternative therapies, no matter how imperfect they may be.
Targets for the Development of Antidepressants
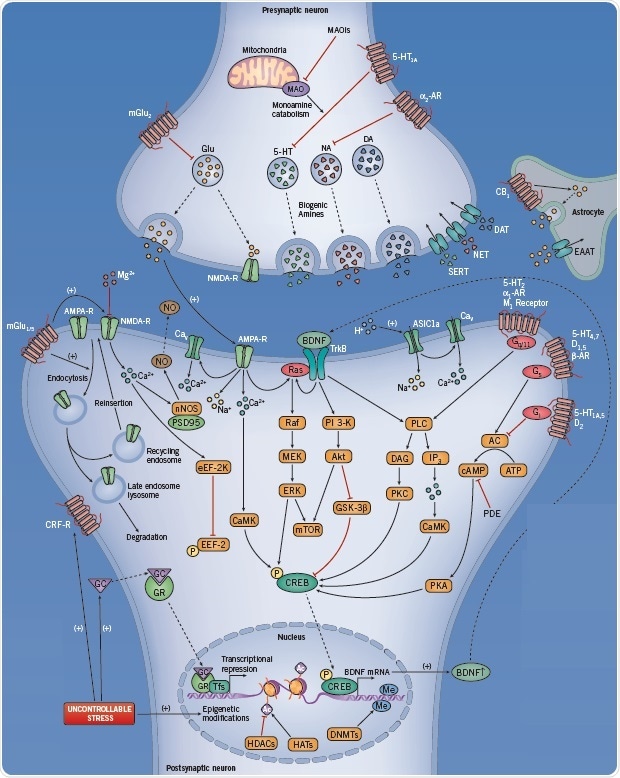
Fig 2. The neurotransmitter pathways involved when suffering from MDD. Image credit: Tocris Bioscience
Presynaptic targets include:
- Receptors that regulate neurotransmitter release, such as the 5-HT1A, α2 adrenoceptor, mGlu2, and NMDA receptors
- MAO, an enzyme that catabolizes monoamines
- Neurotransmitter transporters, exemplified by biogenic amine transporters. Inhibiting these transporters increases the availability of neurotransmitter at the synaptic cleft, a key action in the mechanism of most currently prescribed antidepressants.
Postsynaptic targets:
Targets for AD action include both ionotropic and metabotropic glutamate receptors. In addition, they also include the signal transduction pathways activated by these receptors. These pathways converge to regulate gene expression, the release of neurotrophic factors, and synaptic plasticity, thereby contributing antidepressant action.
mGlu1/5 receptors regulate the function of NMDA receptor by modulating its trafficking and cell surface expression. At resting membrane potential, the NMDA receptor is blocked by Mg2+. However, when the membrane is depolarized, the above block is relieved, thus allowing the entry of Ca2+ into the cell. Ca2+ participates in several cellular cascades, including nitric oxide synthesis, thereby functioning as a retrograde neuronal messenger.
Ca2+ can also enter the cell via voltage-dependent Ca2+ channels (Cav), and subsequently activate Ca2+/calmodulin-dependent protein kinase (CaMK). Cav function can be modulated by ion channels sensitive to glutamate, including the AMPA receptor. Both AMPA receptors and the TrkB receptor (i.e. the receptor for BDNF) have a potential to influence the activity of signal transduction pathways, such as the Ras-Raf-MEK-MAPK and PI 3-K-Akt-GSK-3β signaling pathways, which may be involved in the action mechanism of many antidepressant drugs.
Other metabotropic receptors, such as 5-HT2 , α1 adrenoceptors, and M1 muscarinic receptors, have the ability to stimulate phospholipase C (PLC) and second messengers through Gq/11 signalling. β-adrenoceptors, D1,2,5 receptors, and 5-HT1A,4,5,7 receptors activate the Gs and Gi proteins. These G-proteins modulate adenylyl cyclase (AC) activity, thus regulating the level of cAMP as well as protein kinase A (PKA) activity.
Drugs that influence phosphodiesterase (PDE) enzymatic activity can also affect cAMP concentrations. Scopolamine is a muscarinic receptor antagonist that inhibits M1-M5 with similar affinity. In fact, studies with both knock-out mice and selective pharmacological agents have demonstrated that the M1 and, to a smaller degree M2, receptor subtypes could be the reason behind the rapid and robust antidepressant effects of this drug.
Many of these signal transduction pathways (CaMK, ERK, GSK-3β) converge in the phosphorylation of transcription factors such as cAMP response element-binding protein (CREB), a transcription factor that binds to DNA sequences (cAMP response elements (CRE)), before inducing transcription and the expression of certain genes in the nucleus of the neuron.
Such transformations in gene expression that are induced by antidepressants have the ability to modify the genetic and epigenetic alterations caused by inescapable life experiences. Further, they can also be modified by other genetic factors, which could possibly be involved in the pathophysiology of depression. For instance, the rapid-acting antidepressants (such as ketamine and scopolamine) have been associated with rapid changes in synaptic morphology, including increases in spine density and spine head diameter.
References
- Kessler, R.C., et al. Annu.Rev.Clin.Psychol. 2007
- Compton, W.M., et al. Am.J.Psychiatry. 2006
- Kessler, R.C., and Bromet, E.J., Annu.Rev.Public Health. 2013
- Skolnick, P., et al. Trends Pharmacol.Sci. 2009
- Drevets, W.C., et al. Biol.Psychiatry 2013
- Witkin, J.M., et al. J.Pharmacol.Exper.Ther. 2014
- Voleti, B., et al. Biol.Psychiatry 2013
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.