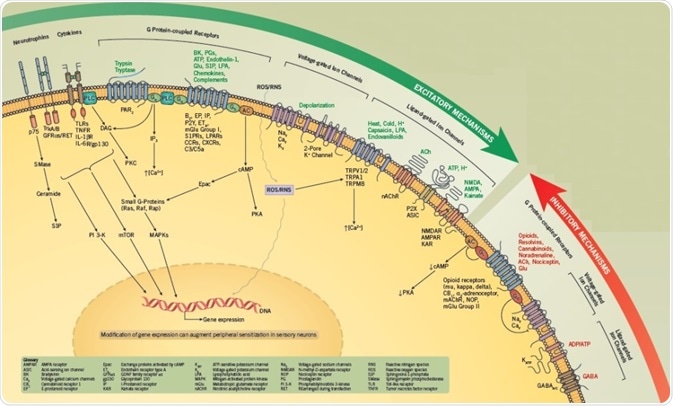
Peripheral sensitization refers to the lowering of the threshold of excitability of sensory neurons, which leads to an amplified reaction to a specific external stimulus.
Sensitization can be mediated, either by post-translational occurrences that change the activity of ion channels and other proteins or through alterations in gene expression that result in a modification of the phenotype of the neuron.
Peripheral sensitization causes a rise in the quantity of neurotransmitter released from peripheral endings of small-diameter sensory neurons and from terminals in the dorsal spinal cord, which increases neurogenic inflammation and perception of pain.
Excitatory Mechanisms
The peripheral endings of primary sensory afferents can be stimulated by noxious stimuli, causing activation of voltage-gated ion channels and/or ligand-gated receptors that result in the production of action potentials.
The transient receptor potential cation channel, subfamily V, member 1 (TRPV1) is the most well-characterized ligand-gated receptor/channel complex and is activated by capsaicin, low pH, heat, and endovanilloids.
Intracellular signaling pathways can be used to modulate the activity of both voltage-gated and ligand-gated channels, meaning that for any chosen stimulus, it is possible to boost the number of evoked action potentials.
This process, called sensitization, can lead to an upsurge in transmitter release from the endings of sensory neurons, thus increasing neurogenic inflammation and/or nociception.
The excitability of sensory neurons is also increased through activation of G protein-coupled receptors (GPCRs) by molecules, including prostaglandins, bradykinin, chemokines, sphingolipids, and other lipids.
Proteinase-activated receptors (PARs), which are activated by enzymatic cleavage of the extracellular N-terminal sequence of the GPCR, also lead to enhanced excitability.
Generally, GPCRs are linked either to Gs, which activate protein kinase A (PKA) or Epacs through cAMP; or to Gq /G11 families, which activate phospholipase C (PLC) to boost intracellular calcium and activate protein kinase C (PKC) isozymes.
Additionally, G protein βγ subunits activate several downstream signaling cascades, including PI 3-Ks and MAPKs, which can cause sensory neurons to become sensitized. Sensitization also occurs as a result of phosphorylation of several target proteins, including ion channels, synaptic proteins, and other kinases.
In adult sensory neurons, nerve growth factor (NGF) enhances sensitivity to thermal and mechanical stimulation via activation of TrkA and/or p75 receptors via signaling cascades, including PI 3-Ks, PLC, Src kinases, MAPKs and the sphingomyelin/ceramide pathway.
The proinflammatory cytokines, tumor necrosis factor, and interleukin-1β (IL-1β) also have significant roles in enhancing the sensitivity of sensory neurons. It is also possible that activation of these signal transduction cascades will directly change gene expression that adds to long-term sensitization.
Inhibitory Mechanisms
A key aim in the development of drugs for pain and inflammation is the ability to reduce peripheral sensitization. In current drug development studies, two approaches have been employed:
- Inhibit the sensitizing actions of proinflammatory agents through synthesis blockers, receptor antagonists, or neutralizing agents (antibodies or pseudoreceptor).
- Decrease excitability through a direct action on inhibitory pathways.
Several antinociceptive agents can diminish excitability and constrain transmitter release through activation of GPCRs.
These GPCRs are generally linked to Gi/Go. Activation of Gi impedes adenylyl cyclases, reduces cAMP content, and minimizes PKA activity. In contrast, pathways for Go may involve the liberation of βγ subunits that directly obstruct channel activity or alter other undefined targets.
To illustrate, the activation of opioid receptors results in hyperpolarization. It is assumed this is a result of the activation of potassium channels and inhibition of calcium channels, both of which play a part in a decrease in transmitter release.
A group of lipids, known as resolvins, can lessen excitability by activating G proteins. Therefore, the ability to decrease the activity of transduction cascades that mediate peripheral sensitization may be found to offer a new therapeutic method through which to treat pain and inflammation.
Activation of certain ion channels can reduce the excitability of primary afferents. Chloride current activated by GABA is the best studied of these, however, the physiological role of the majority of inhibitory channels is not well understood.
Conversely, great attention has been paid to the inhibition of excitatory ion channels. Local anesthetics characterize a key class of drugs that offer pain relief through blocking the activity of voltage-dependent sodium channels, inhibiting the generation of the action potential.
In recent times, blockers of the calcium channel CaV 2.2 are effective antihyperalgesic agents.
References and Further Reading
- Beggs and Salter (2010) Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr. Opin. Neurobiol. 20 474.
- Nicol and Vasko (2007) Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol. Interv. 7 26.
- Park and Vasko (2005) Lipid mediators of sensitivity in sensory neurons. Trends Pharmacol. Sci. 26 571.
- Salvemini et al (2013) Therapeutic targeting of the ceramide-to-sphingosine 1-phosphate pathway in pain. Trends Pharmacol. Sci. 34 110.
- Scholz and Woolf (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 10 1361.
- von Hehn et al (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 73 638.
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.