Recognition memory allows judgments to be made about whether a particular stimulus has been encountered before or not. Recognition memories are promptly acquired at the time of a single encounter and are important to lead to a normal life. Deterioration in recognition memory is the main symptom of the typical amnesia and early Alzheimer's disease. A lot of the same neurotransmitter receptors and intracellular signaling cascades that result in synaptic plasticity (i.e. long-term depression; LTD) in the perirhinal cortex are also essential for perirhinal cortex-dependent recognition memory.
Role of the Perirhinal Cortex
The perirhinal cortex is present within the medial temporal lobe (Figure 1) and it is considered to play a vital role in recognition memory, and also in perception and object association. Studies have reported that up to 25% of perirhinal cortex neurons exhibit a reduced response to a visual stimulus when it is displayed for a second or subsequent time. Neuronal modeling and experimental evidence have proposed that this decrease in synaptic strength facilitates the neural mechanism through which the awareness of an object may be encoded within the perirhinal cortex.
Furthermore, the role of the perirhinal cortex in memory and the connections between synaptic plasticity and recognition memory, have been described by means of pharmacological infusion studies. The following experimental data were obtained from rodent models.
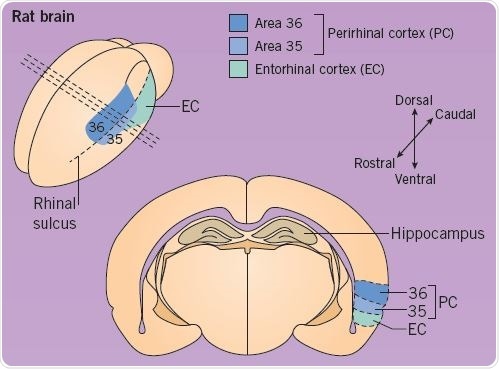
Fig 1. Location of the Perirhinal Cortex in a rat brain. Image credit: Tocris Bioscience
Recognition Memory in Rodents
Recognition memory may be tested in rodents with the help of single-trial object memory tasks. Rodents are permitted to look at an object for a specific period of time in the sample phase, and in a subsequent test phase, the animals are presented with a new object and the known object (Figure 2). In the test phase, normal rodents prefer to look at the new object, thereby demonstrating familiarity discrimination; this denotes that their recognition memory for the familiar object is intact. Such spontaneous object recognition memory tasks do not need reinforcement or long training sessions, and closely resemble tasks that measure human recognition memory.
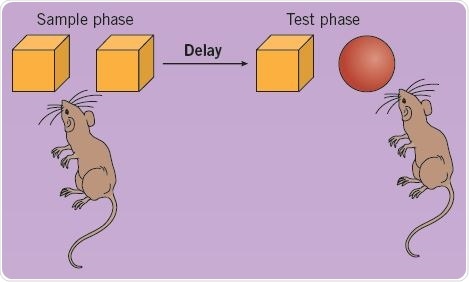
Fig 2. Using a single-trial object memory task to test recognition memory in rats. Image credit: Tocris Bioscience
Pharmacological Intervention
The role of various neurotransmitters in recognition memory may be evaluated through pharmacological intervention. Antagonists are injected directly into the rodent’s perirhinal cortex through indwelling cannulas. As shown in the figure, administration of the muscarinic receptor (mAChR) antagonist scopolamine (0.05 ng/μL) weakens recognition memory at a 20-minute delay, but not 24-hour delay (*p<0.05). In contrast, administration of the nicotinic receptor (nAChR) agonist methyllycaconitine (MLA) weakens memory at a 24-hour delay, but not 20-minute delay.
The discrimination ratio is an indicator of memory performance and is estimated as the difference in the amount of time taken to explore the new and known objects as a proportion of total exploration time (Figure 3; adapted from Tinsley et al (2011)).
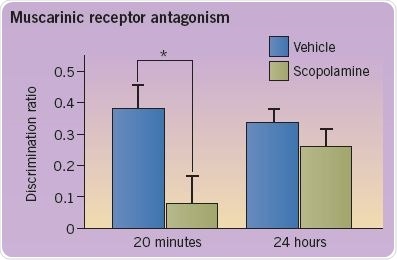
Fig 3. A graph depicting the discrimination ratio to demonstrate memory performance. Image credit: Tocris Bioscience
Elucidating the Mechanisms of Long-term Depression
In addition, the initiation of LTD in the perirhinal cortex is inhibited by scopolamine. Electrophysiological recordings obtained from slices of the perirhinal cortex are used to evaluate synaptic plasticity. A graph illustrating the amplitude of evoked synaptic responses is shown below (left). LTD is experimentally brought about by conditioning stimulation (represented by two upward arrows). Administration of scopolamine (right) inhibits this induction; there, it is believed that there is a close correlation between recognition memory and LTD. The following traces are representative EPSCs taken from the time points specified (1 and 2) (Figure 4; adapted from Cho et al (2000) and Warburton et al (2003)).
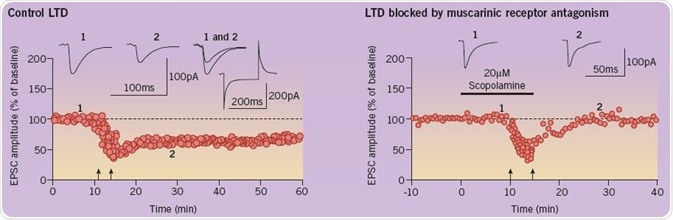
Fig 4. A comparison of the amplitude of evoked synaptic responses in the control group and the group with blocked LTD. Image credit: Tocris Bioscience
Cellular Mechanisms of Long-term Depression and Recognition Memory
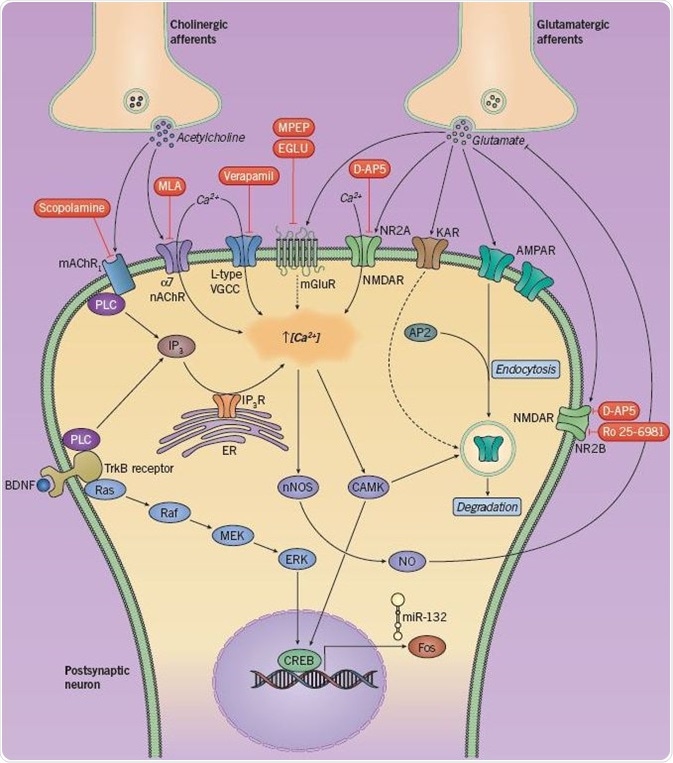
Fig 5. Neurotransmitters and signaling cascades involved in LTD and recognition memory. Image credit: Tocris Bioscience
Several neurotransmitters and intracellular signaling cascades have been known to play a role in LTD as well as recognition memory (Figure 5). The discharge of acetylcholine and glutamate from presynaptic afferents triggers an array of postsynaptic receptors. Stimulation of glutamate receptors (mGluR, NMDAR, AMPAR) and cholinergic receptors (nAChR and mAChR) induces an influx of Ca2+ ions, as does the stimulation of L-type voltage-gated calcium channels and TrkB receptors.
The resultant rise in intracellular Ca2+ concentration triggers the activation of calcium-calmodulin related kinases (CAMKs), leading to the phosphorylation of both the transcription factor CREB and AMPA receptors (causing endocytosis and LTD). CREB is also phosphorylated due to a MAPK signaling cascade triggered by BDNF/TrkB. CREB can upregulate the instantaneous early gene c-Fos and regulates the production of the microRNA miR-132; however, the mechanisms by which they sustain LTD are unknown.
It is assumed that the increase in intracellular Ca2+ also stimulates neuronal nitric oxide synthase (nNOS), leading to the synthesis of nitric oxide (NO), which consecutively blocks the discharge of glutamate and makes up a type of negative feedback. Expression of LTD can be regulated via internalization of synaptic AMPA receptors, a process that is dependent on the communication between the AP2 protein and AMPA receptors; the former is an adaptor protein that plays a role in clathrin-mediated endocytosis.
Kainate receptor (KAR) activation also leads to a variation in synaptic strength, possibly as a result of its participation in AMPAR endocytosis. Pharmacological inhibition (in red) of glutamatergic or cholinergic receptors has been established to weaken either short- or long-term recognition memory.
References
- Barker et al (2006) J. Neurosci. 26 3561
- Barker et al (2006) Learn. Mem. 13 178
- Cho et al (2000) Nat. Neurosci. 3 150
- Griffiths et al (2008) Neuron 58 486
- Massey et al (2004) J. Neurosci. 24 7821
- Scott et al (2012) Eur. J. Neurosci. 36 2941
- Seoane et al (2009) J. Neurosci. 29 9534
- Seoane et al (2011) Hippocampus 13 178
- Seoane et al (2012) Hippocampus 22 2101
- Tinsley et al (2011) Learn. Mem. 18 484
- Warburton et al (2003) Neuron 38 987
- Warburton et al (2005) J. Neurosci. 25 6296
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.