Heart failure, also referred to as congestive heart failure or CHF, corresponds to the inability of the heart to pump enough blood around the body. Heart failure usually transpires secondary to an existing pathology that modifies cardiac function. Myocardial infarction, arrhythmia or infection are some examples of syndromes that can precede heart failure. These can also lead to dilated cardiomyopathy, a condition accounting for roughly a third of all heart failure cases.
Pathogenesis of Heart Failure
The pathogenesis of heart failure is progressive and cyclical; endogenous mechanisms, which undergo activation during heart failure to frustrate the symptoms, instead reduce cardiac function. Cardiac dysfunction, either diastolic or systolic, engenders a lowering of stroke volume and a consequent lowering of cardiac output. Among healthy individuals, the body reacts to decreases in cardiac output by activating the renin-angiotensinaldosterone system as a means of promoting fluid retention, and also by initiating the sympathetic nervous system as a means of engendering peripheral vasoconstriction.
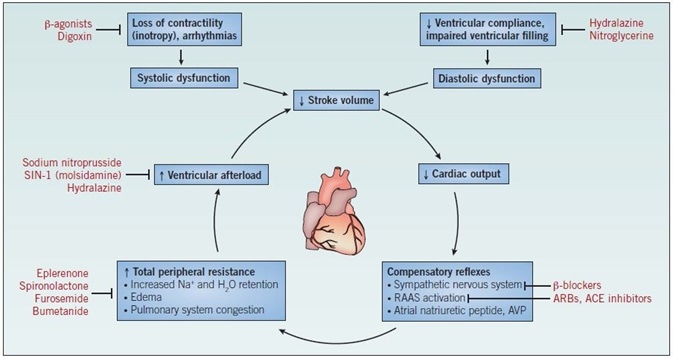
Figure 1. The mechanism of heart failure. Abbreviations: ACE – angiotensin-converting enzyme; ARBs – angiotensin II receptor blockers; AVP – arginine vasopressin; RAAS – reninangiotensin- aldosterone system.
In ordinary circumstances, this counteracts the imbalance in stroke volume, causing restoration of cardiac output to ordinary levels. Among patients with heart failure, the increase in blood volume, in conjunction with the elevated peripheral resistance and heightened levels of circulating catecholamines, leads to an intensified load on the already weakened ventricles after each contraction, with the stroke volume not returning to normal levels. Repeated cycles of this mechanism cause further weakening of the ventricle walls, engendering ventricular hypertrophy and a lowered force of contraction (Figure 1).
Although surgical intervention, which includes the implantation of a left ventricular assist device, is available for patients with heart failure, these are invasive and unsuitable for some patients. Pharmacological intervention is common and supported by the range of available drugs which target the various stages of the heart failure process. Nevertheless, none of these extant therapeutic strategies can reverse the pathology of heart failure and function only as a means of slowing the progression of the disease.
Common Pharmacological Therapies
The two most common pharmacological therapies for heart failure comprise increasing intracellular calcium concentration within myocytes via the activation of second messenger signaling pathways; and blocking or counteracting of the neurohormonal compensatory reflexes via the inhibition of the RAAS.
Pharmacological agents triggering an increase in intracellular calcium comprise β adrenergic receptor agonists; the positive inotrope digoxin, an Na+/K+ ATPase blocker; and phosphodiesterase inhibitors. Unfortunately, the indirect targeting of the signaling pathways implicated in cardiac contractility, in turn, engenders mechanism-related adverse effects. Contemporary therapy, which uses small molecule drugs, including the cardiac myosin activator, omecamtiv mecarbil, represents an encouraging, more effective strategy for augmenting contractility among patients with heart failure.
In comparison to increasing contractility, blocking the neurohormonal reflexes in the failing heart, achieved using ACE inhibitors such as perindopril, or ARBs including valsartan, is a much more effective therapeutic strategy for heart failure. Administering diuretics lessens symptoms connected to heart failure, such as peripheral edema, but it does not reverse or stop the disease pathology. Furosemide, an NKCC co-transporter inhibitor, and spironolactone, an aldosterone receptor antagonist, represent two examples of diuretics utilized in the treatment of heart failure.
Therapeutic Target for Heart Failure
Vasodilators, including hydralazine, nicorandil and SIN-1, are also utilized in the therapy of heart failure as they cause peripheral vasodilation, and thus reduce ventricular afterload. As well as the extant compounds, future therapeutic targets comprise the collagenase enzymes MMP-2 and MMP-9. The expression of these metalloproteinases is augmented in heart failure, whereas the expression of their endogenous inhibitors, tissue inhibitors of metalloproteinases (TIMPs), has been proven to undergo downregulation in the same tissue. To reinforce this, experimental inhibition of MMP-9 lowers ventricular dilatation in a model of heart failure. An additional therapeutic target in heart failure and one which is critical to disease progression is the activity of G protein-coupled receptor kinases (GRKs), particularly the cardiomyocyte-expressed GRK2. GRK2 activation during heart failure causes the desensitization of β adrenergic receptors (βARs), thus reducing contractility and depressing cardiac function.
Circulating levels of GRK2 and GRK5 undergo upregulation during the initial stages of heart failure, while both cardiac isoforms of βARs – β1ARs and β2ARs – have been proven to undergo downregulation, becoming non-functional. Cardiomyocyte-specific overexpression of GRK2 caused βAR uncoupling, leading to a reduction in contractility, while the expression of an inactive form of GRK2 in either adrenal or cardiac triggered an increase in contractility in response to adrenergic stimulation. Consequently, cardiomyocyteor adrenal-specific inhibition of GRK2 might constitute a novel therapeutic target for heart failure.
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.