Tumor metastasis is a multistep process that involves the distribution of tumor cells from the primary tumor to a different organ or tissue. For metastasis to take place, the tumor has to infiltrate the extracellular matrix (ECM) and the surrounding stroma and go through a process known as epithelial-mesenchymal transition (EMT).
Epithelial-Mesenchymal Transition
EMT facilitates cell mobility and the migration of epithelial cells that have gained mesenchymal characteristics, notably the loss of adherins (specifically E-cadherins) and the loss of cell polarity. The cell then intravasates into the blood or lymphatic vessels to then become a circulating tumor cell (CTC). The CTC eventually settles at a new site, for example in the liver or lung, and goes through mesenchymal-epithelial transition (MET), and attaches to the new tissue. Then, the tumor cell reinitiates the process of growth and division, developing into a new tumor called “metastatic colonization”.
Degradation of Extracellular Matrix
The degradation of the basement membrane is carried out by matrix metalloproteases (MMPs), which are secreted directly from the tumor cells or by surrounding stromal cells that are being stimulated by the neighboring tumor. Several studies have implicated altered MMP expression in a range of human cancers that carry a poor prognosis. MMP-1, -2, -3, -7, -9, -13 and -14 all have elevated expression in primary tumors and metastases. Both synthetic and natural inhibitors of MMPs halt metastasis, while upregulation of MMPs results in increased cancer cell invasion.
Other proteases, such as urokinase (uPA), are also involved in ECM degradation (Figure 1). This breakdown in the integrity of the matrix creates a route for the tumor cells to make their way into the bloodstream or lymphatic system.
Upregulation of certain receptor tyrosine kinase (RTK) signaling pathways can aid both invasion and metastasis. For example, epidermal growth factor receptor (EGFR), transforming growth factor-β (TGF-β) receptor, and the MET receptor, also known as hepatocyte growth factor receptor (HGFR), can all mediate initiation signals that increase Snail transcription. Heightened levels of the transcription factor Snail downregulate E-cadherin transcription and promoting EMT.
The endogenous ligand for c-MET is hepatocyte growth factor/scatter factor (HGF), a molecule created mainly predominantly mesenchymal cells. This explains why MET receptor signaling is the main element working towards invasive growth and EMT. Aberrant activation of the HGF/MET pathway leads to a wide range of different cancers and has been linked with a poor disease prognosis because it can facilitate tumor growth, the growth of new blood vessels, and metastasis. Two key research tools for studying EMT include crizotinib and SU 11274.
Inhibitors of Mesenchymal-Epithelial Transition
Crizotinib is a powerful inhibitor of c-MET (and ALK) that shows antitumor values in several different cancer models. Selectively, crizotinib inhibits c-MET-dependent proliferation, migration, and invasion of human tumor cells in vitro. It could be proved useful in vivo because it is bioavailable orally. SU 11274 is a selective inhibitor of MET tyrosine kinase activity that reduces cell growth and triggers cell cycle arrest and cell death. Additionally, it impedes cell motility and migration in vitro and tumor angiogenesis in vivo.
Disruption or loss of adhesive molecules such as cadherins and integrins, integral elements in cell-cell adhesion, and cell-ECM interactions respectively, play an essential role in metastasis. This is because they enable tumor cells to start to develop metastatic colonies at a second distant site. Reduction in E-cadherin expression is a key player in EMT and increases the chances of metastatic cancer cell dissemination.
Cellular Adhesion Inhibitors
Compounds such as BMS 536924 reverse EMT by hindering Snail-mediated downregulation of E-cadherin. Integrin receptors ‘integrate’ the extracellular environment with the cell interior by binding both the extracellular matrix (ECM) and the cytoskeleton. They are essential for cells to attach to the ECM, mediated through integrin-fibronectin, -vitronectin, -collagen and -laminin interactions. BIO 1211 and BIO 5192 are selective and potent α4 β1 integrin receptor inhibitors, which could prove to be valuable tools for investigating the role of integrin receptors in metastasis.
Focal adhesion kinase (FAK) is also involved in cellular adhesion. It is activated in response to integrin-ECM interactions, becoming a critical focal point for several signaling components involved in cell growth and motility. There are numerous potent and selective FAK research compounds, from FAK Inhibitor 14 and PF 431396, to PF 573228.
FAK Inhibitor 14 promotes the detachment of cells and impedes cell adhesion in vitro, and shows antiproliferative activity in numerous human tumor cell lines in vitro and in breast cancer cells in vivo. PF 431396 is a dual (FAK) and proline-rich tyrosine kinase 2 (PYK2) inhibitor, which is a useful area for an investigation into cell migration, as PYK2 is also a key mediator of cell migration and proliferation. PF 573228 is a powerful and selective inhibitor of FAK that blocks serum and fibronectin-directed migration and decreases focal adhesion turnover in vitro.
FAK activates the Rho-family GTPases (Rac, RhoA and Cdc42). This family regulates actin assembly and the stability of microtubules involved in cell migration.
A multitude of direct inhibitors is part of the Rho-family GTPases, including EHT 1864, while others like SC 23766, mediate their actions by interrupting Rac1 interactions.
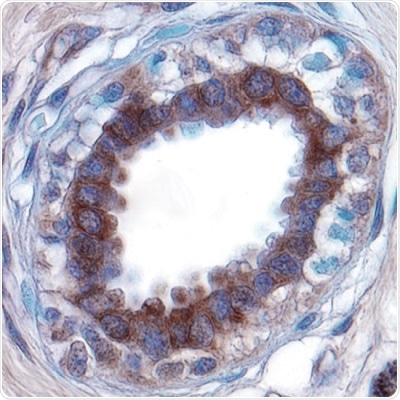
Figure 1: Extracellular Matrix Degradation.
Urokinase-type Plasminogen Activator (uPA) expression detected in paraffin-embedded sections of human breast cancer tissue. uPA is a serine protease that is involved in ECM degradation, resulting in a loss of matrix integrity and a potential route through which tumor cells can to migrate to other tissues. Visualized here in brown using a Goat Anti-Human/Mouse uPA Affinity-purified Polyclonal Antibody (R&D Systems, Catalog #AF1310). Hematoxylin counterstain in blue.
NSC 23766 is a selective inhibitor of the Rac1-GEF (guanine nucleotide exchange factor) interaction. This compound stops Rac1 from activating with GEFs TrioN and Tiam1 without affecting the activation of Cdc42 or RhoA. Additionally, this compound has been found to reverse tumor cell phenotyes in prostate cancer cells.
RhoA activates Rho-associated protein kinase (ROCK), which then regulates cell proliferation and mediates tumor cell migration by acting on the cytoskeleton. Preclinical studies have shown that Combination treatment with RTK inhibitors can inhibit hematological malignancies, as has been shown in preclinical studies, and additional early studies have shown that classic ROCK inhibitors such as Y-27632 and fasudil were able to impede metastasis in in vivo cancer models. Additionally, fasudil was also proved to suppress MMP-2 expression and bring on cell death in glioblastoma cells in vivo. Other notable ROCK inhibitors include the potent and selective ROCK inhibitors GSK 269962 and GSK 429286, either of which may prove to be useful research tools for future investigations into the role of ROCK in cancer models.
Found downstream of Rac1 and Cdc42 is group I p21-activated kinases (PAKs 1-4). These molecules link Rho GTPases with cytoskeletal remodeling and cell motility. Recently, they have also been found to encourage cell proliferation and regulate cell death. Both overexpression and abnormal regulation of PAKs promote the formation of tumors. IPA 3 promotes the inactive conformation of PAKs and impedes PAK1-mediated signaling in vivo, potentially showing antitumor activity.
Overexpression of Liver receptor homolog-1 (LRH1) promotes motility and invasiveness in ER+ and ER– breast cancer cells by remodeling the cytoskeleton, and by facilitating posttranslational modifications to E-cadherin and promoting EMT.
Overexpression of LRH1 has also been associated with poor disease prognosis in liver cancer, pancreatic cancer, and gastric cancer. It can be hypothesized that compounds like the selective LRH1 inverse agonist ML179 and the selective agonist DLPC could be useful tools in the investigation of tumor cell migration and invasion.
Conclusion
Metastasis is often closely related to disease outcomes or clinical prognosis. The mechanisms by which this process works have been of particular interest in cancer research as a result. The development of new pharmacological tools, in particular, has helped illuminate the cellular changes and molecules involved in triggering the invasion of tumor cells and metastasis. Research in the future may also take into account the roles immune cells play and tumor metabolism in the dynamics of metastasis.
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.