The gut is the most sizeable endocrine organ in the body. Over thirty hormones are generated by the gastrointestinal tract, pancreas, and fat, with several additional, related peptides generated in the brain. A high proportion of gut hormones are released through the direct action of ingested nutrients on enteroendocrine cells located within the intestine. These hormones function to manage food intake and energy expenditure.
Gut-Brain Axis
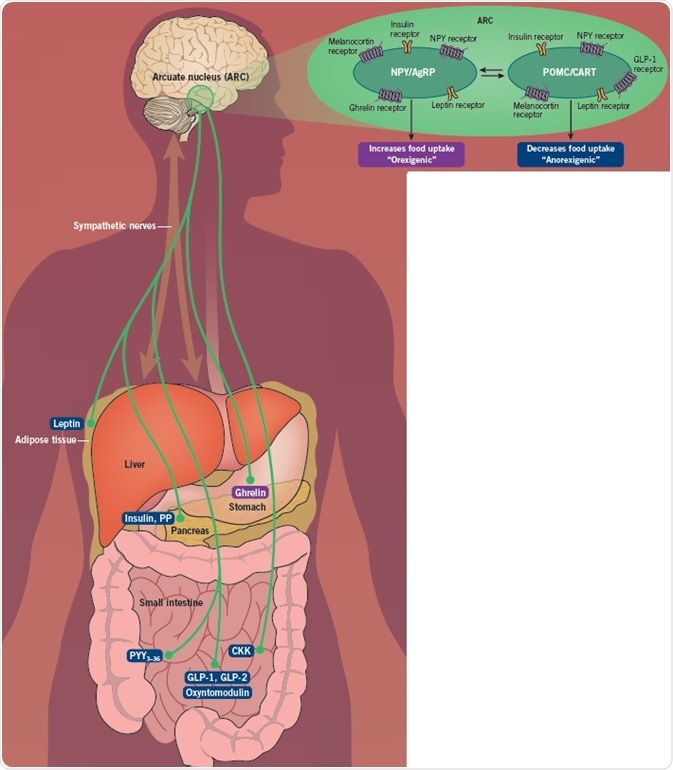
The hypothalamus is the coordination center for energy homeostasis, while the arcuate nucleus (ARC) in the hypothalamus is the epicenter for the integration of signals regarding an individual's energy status and requirements.
The ARC comprises two distinct populations of neurons. AgRP and NPY neurons are orexigenic (stimulate appetite) and are stimulated by signals like ghrelin, whereas POMC and CART neurons are anorexigenic (reduce appetite), and are activated by PYY and GLP-1. These neurons undergo reciprocal innervation of each other, so the AGRP/NPY neurons are turned off when the POMC/CART neurons are activated, and vice versa.
The ARC receives inputs from numerous divergent sources. It exists near to the median eminence, which lacks a blood-brain barrier, permitting direct access to hormones from the periphery. The vagus nerve connecting the hypothalamus to the gastrointestinal tract relays messages regarding gastrointestinal distension and gut hormones. Reciprocal connections also occur between the hypothalamus and the brain stem.
The ARC subsequently relays messages to further hypothalamic nuclei, including the dorsomedial nucleus, the lateral hypothalamic area, and the ventromedial nucleus. There is then output to the sympathetic nervous system, the thyroid axis, the limbic system, and back to the vagus, which subsequently manages food intake and energy expenditure.
Gut Hormones and Obesity
In typical circumstances, coordination of the gut-brain axis allows an individual to maintain their weight within a narrow range. In contrast, continuous immoderate food consumption can override the usual homeostatic mechanisms and cause the development of obesity. Moreover, as soon as a person has become obese, their physiology is adjusted, making weight loss even more difficult.
Among obese patients, the levels and efficacy of the satiety hormones PP, GLP-1, CCK, and PYY undergo a relative reduction. Moreover, there develops an increased sensitivity to ghrelin, alongside resistance to the effects of leptin.
These alterations prevent the feeling of being full, increasing food consumption as a result. Moreover, the body attempts to fight against weight loss during dieting. There is a drop in satiety hormones (such as leptin, amylin, CCK and PYY), whereas the orexigenic ghrelin and NPY increase. The metabolic rate also decelerates, which means it becomes increasingly more difficult to lose weight and maintain weight loss.
| Hormone |
Obesity |
Weight loss |
| PYY |
↓ post-prandial rise |
↓ |
| PP |
↓ / ↑ |
↓ / ↑ |
| GLP-1 |
↓ post-prandial rise |
↓ |
| CCK |
↓ / ↑ |
↓ |
| Leptin |
Increased baseline levels but increased resistance to action |
↓ |
| Ghrelin |
Reduced baseline levels and failure to suppress post-prandially |
↓ |
| Amylin |
↑ |
↓ |
Gut Hormones, Drug Therapies, and Bariatric Surgery
Gut hormones can be utilized as a pharmacological treatment for obesity. Although naturally occurring gut hormones possess very brief half-lives in the body, thus limiting their usage, long-lasting versions are now undergoing development.
Exendin-4 is a GLP-1 analog initially located in the saliva of the Gila monster. It embodies resistance to the enzyme dipeptidyl peptidase IV, which breaks down GLP-1, and thus possesses a prolonged half-life. Exenatide, the synthetic version, is regularly used as a therapy for diabetes. Liraglutide, an additional long-acting GLP-1 analog, is used as a therapy for both obesity and diabetes.
Stabilized analogs of both PP and PYY have undergone development, entering clinical trials as therapies for obesity. There is growing evidence for tackling obesity with mixtures of gut hormones.
Chronic injections of oxyntomodulin, which stimulates both glucagon and GLP-1 receptors, lower body weight among obese patients, and several oxyntomodulin analogs are being developed as a therapy for obesity. In addition, a number of drugs are being developed that target both the GIP and GLP-1 pathways, and triple-agonists at the glucagon, GIP, and GLP-1 receptors.
Today, bariatric surgery is acknowledged to be the most effectual, enduring therapy for obesity. There are two consequences of surgery. First of all, the size of the GI tract, especially the stomach, is reduced, causing patients to eat less. The levels of gut hormones are also altered, fostering a more anorexic environment.
Roux-en-Y bypass is the most typical category of bariatric surgery, following which the post-prandial response is changed so that PPY, GLP-1, CCK, glucagon, and oxyntomodulin, are all increased, whereas ghrelin and GIP levels drop. Although there is a drop in gastrin levels, these are increased by PPI treatment. There is no notable modification in PP levels. The alterations in gut hormones, especially GLP-1, might bring about the improvement in diabetes observed following bariatric surgery, independent of weight loss.
Orexigenic Mediators
| Hormone |
Release From |
Receptor |
| Agouti-related peptide (AgRP) |
Hypothalamus, particularly arcuate nucleus |
Inverse agonist of Melanocortin MC3 and MC4 receptors |
| Endocannabinoids |
Central nervous system |
CB1 and CB2 receptors |
| Galanin |
Enteric neurons, central and peripheral nervous system |
G-protein coupled receptors GAL1, GAL2 and GAL3 |
| Ghrelin |
X/A-like cells of the stomach |
Ghrelin receptor (also increases preference for sweet food) |
| Growth hormone-releasing hormone (GHRH) |
Hypothalamus |
Growth hormone-releasing hormone receptor |
| Melanin-concentrating hormone (MCH) |
Hypothalamus |
Melanin-concentrating hormone receptor |
| Neuropeptide Y (NPY) |
Hypothalamus and enteric neurons |
Y1, Y2 and Y5 receptors (increases food intake via Y1 and Y5; decreases food intake via Y2 receptor) |
| Orexin B |
Intestine and hypothalamus |
OX1 and OX2 receptors |
Anorexigenic Mediators
| Hormone |
Release From |
Receptor |
| Amylin |
β-cells of the pancreas |
AMY1a, AMY2a, and AMY3a (Calcitonin receptor core, with associated receptor activity modifying protein RAMP1, RAMP2 or RAMP3) |
| Calcitonin gene-related peptide (CGRP) |
Enteric neurons, central and peripheral nervous system |
CGRP receptor (Calcitonin receptor-like receptor with associated RAMP1) |
| Cholecystokinin (CCK) |
I-cells duodenum |
CCK1 and CCK2 receptors |
| Cocaine and amphetamine-regulated transcript (CART) |
Hypothalamus |
The CART receptor has not been fully identified |
| Corticotrophin-releasing hormone (CRH) |
Hypothalamus |
CRHR1 and CRHR2 receptors (reduces or increases food intake depending on route of administration) |
| Gastrin releasing peptide |
Enteric neurons and the central nervous system |
BB2 receptor |
| Glucagon |
α-cells of the pancreas |
Glucagon receptor |
| Glucagon-like peptide 1 |
L-cells of the ileum |
GLP-1 receptor (also reduces preference for sweet food) |
| Glucagon-like peptide 2 |
L-cells of the ileum |
GLP-2 receptor |
| Glucose-dependent insulinotropic peptide |
K-cells of the jejunum |
GIP receptors |
| Insulin |
β-cells of the pancreas |
Insulin receptor |
| Leptin |
Adipose tissue |
Leptin receptor |
| α-melanocortin-stimulating hormone (α-MSH) |
Hypothalamus |
Melanocortin MC3 and MC4 receptors |
| Neuromedin B |
Hypothalamus and enteric neurons |
BB1 receptor |
| Neuromedin U (NMU) |
Central nervous system and intestine |
NMU1 and NMU2 receptors |
| Neurotensin |
Central nervous system and N cells of the intestine |
NTS1 and NTS receptors |
| Opioid Peptides (met-enkephalin, leu-enkephalin, β-endorphin and dynorphin) |
Enteric neurons |
μ, κ and δ-opioid receptors |
| Oxyntomodulin |
L-cells of the ileum |
Co-agonist of glucagon and GLP-1 receptors |
| Pancreatic polypeptide |
PP-cells of the pancreas |
Y4 receptor |
| Peptide tyrosine tyrosine (PYY3-36) |
L-cells of the ileum |
Y2 receptor |
| Pituitary adenylate cyclase-activating polypeptide (PACAP) |
Intestine and nervous system |
PAC1, VPAC1, and VPAC2 |
| Urocortin |
Brain and widely distributed in the periphery |
CRF receptors |
| Vasoactive intestinal polypeptide |
Intestine and nervous system |
VPAC1 and VPAC2 |
References and Further Reading
- Lean and Malkova (2016) Int. Journal of Obesity (London) 40 622
- Meek et al (2016) Peptides 77 28
- Pi-Sunyer et al (2015) NEJM 373 11
- Troke et al (2014) Ther. Adv. Chronic Dis. 5 4
- Wilson and Enriori (2015) Mol. Cell Endocrinology 418 108
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.