Across the world, cancer is a growing health concern. So far, the most common cancer treatments have been chemotherapy, surgery and radiation therapy. Cancer immunotherapy has also become a hopeful new treatment option for several types of cancer.
Chimeric antigen receptor T-cell therapy is known as a revolutionary new pillar of cancer treatment since it generates considerable and durable clinical responses. Chimeric antigen receptors (CARs, also called chimeric immune receptors) are receptor proteins altered to provide T-cells with new potentials to aim at specific proteins.
Such receptors are chimeric because they integrate antigen binding and T-cell activation functions into a single receptor. Once injected into a patient, CAR T-cells serve as a so-called “living drug” against cancer cells.1
When they come into contact with the targeted antigen on the cell, CAR T-cells tend to bind to it and are activated, then they continue to proliferate and produce cytotoxicity (Figure 1).2
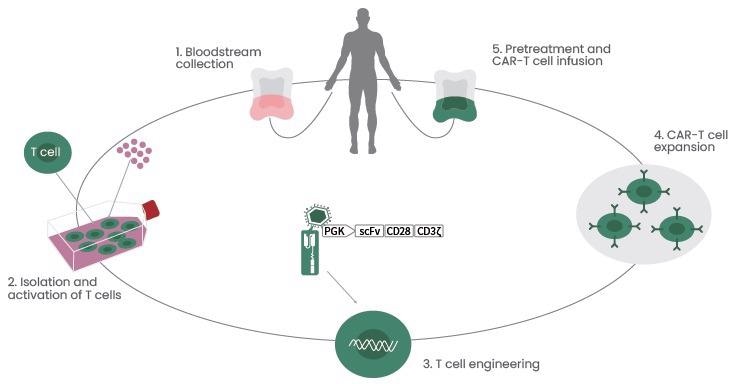
Figure 1. CAR T-cell Therapy Procedures. Image Credit: Sino Biological Inc.
Four iterations have been experienced by CAR T-cells. The initial CAR T-cells were developed in 1987 by Kuwana, after the second, third, and fourth-generation compositions.
Effector function, antitumor activity, and in vivo persistence with extended enhancement modifications on such generational compositions enable enzymatic degradation of the extracellular matrix by solid tumors and costimulation of numerous receptors with extra ligands.3
As a result, two landmark events for CAR T-cell therapy have taken place in 2017: the Food and Drug Administration (FDA) approved two anti-CD19 CAR T-cells to treat acute lymphoblastic leukemia (ALL) and relapsed or refractory large B-cell lymphoma, respectively.
So far, there are currently six products of CAR T-cell therapies that have been approved by the FDA (Table 1).
Table 1. FDA-approved CAR T-cell therapies. Source: Sino Biological Inc.
| CAR T-cell |
Brand name |
Approval date |
Target |
Indication (Targeted disease) |
| tisagenlecleucel |
Kymriah |
08/30/2017 |
CD19 |
B-cell acute lymphoblastic leukemia
Diffuse large B-cell lymphoma (DLBCL) |
| axicabtagene ciloleucel |
Yescarta |
10/18/2017 |
CD19 |
Diffuse large B-cell lymphoma (DLBCL)
Follicular lymphoma |
| brexucabtagene autoleucel |
Tecartus |
07/24/2020 |
CD19 |
Mantle cell lymphoma (MCL) |
| lisocabtagene maraleucel |
Breyanzi |
02/05/2021 |
CD19 |
Diffuse large B-cell lymphoma (DLBCL) |
| idecabtagene vicleucel |
Abecma |
03/26/2021 |
BCMA |
Multiple myeloma |
| ciltacabtagene autoleucel |
Carvykti |
02/28/2022 |
BCMA |
Multiple myeloma |
CAR T therapy in tumors
By adopting genetic modification technology, CAR T-cell immunotherapy has greater targeting, killing activity and persistence of effector T-cells compared to traditional immune cells.
Also, it can overcome the tumor’s local immune suppression microenvironment and crack the host immune tolerance state, displaying huge application potential and development possibilities in the clinical treatment of tumors.
But there are also threats of cytokine storm, insertion mutation, off-target effect and other clinical applications.
CAR T-cell therapy in hematologic cancer
CAR T-cell therapy has benefitted an initial foothold in blood-borne cancers. CAR T-cell-based therapies with high efficacy are extensively utilized in hematological cancers like acute and chronic forms of lymphoma, myeloma and leukemia.
Anti-CD19 CAR T-cells are efficient for treatingR/R (relapsed or refractory) B-cell malignancies, like B-cell non-Hodgkin lymphoma (NHL), ALL, and chronic lymphocytic leukemia in both pediatric and adult patients4. CD19, a member of the immunoglobulin superfamily expressed on the surface of B cells, acts as a perfect target for car-targeted therapy.5
In Phase 2 of the multicenter ELIANA trial, a full remission rate of 60% in 75 children and young adults, a full remission rate of 81%, a durable remission rate of 80%, and a 6-month relapse-free survival was linked to extended CAR T-cells in peripheral blood samples and sustained B-cell proliferation.6
B-cell maturation antigen (BCMA) belongs to the tumor necrosis factor receptor superfamily controlling B-cell proliferation and survival.
BCMA is known to be a highly selective expression in malignant plasma cells. It has become the main target for bispecific antibodies, chimeric antigen receptor T-cell therapies, antibody-drug conjugates, and other immunotherapies in multiple sclerosis myeloma.7
The CD22-CAR T-cell could target one more overexpressed antigen on B-cell tumors, CD22. The impressive antitumor function of CD22-CAR T cell was noted in relapsed cancer following CD19-CAR T-cell therapy or in an era guiding ALL tumor cells.
Furthermore, various studies have verified the safety and efficiency of CD20-CAR T-cells in R/R NHL, follicular and set-in-cell lymphoma.8
Sino Biological offers numerous recombinant hematologic tumor target proteins, ELISA Kits, and more (Figure 2). Several techniques have confirmed these high-quality reagents and will offer constant and reproducible outcomes.
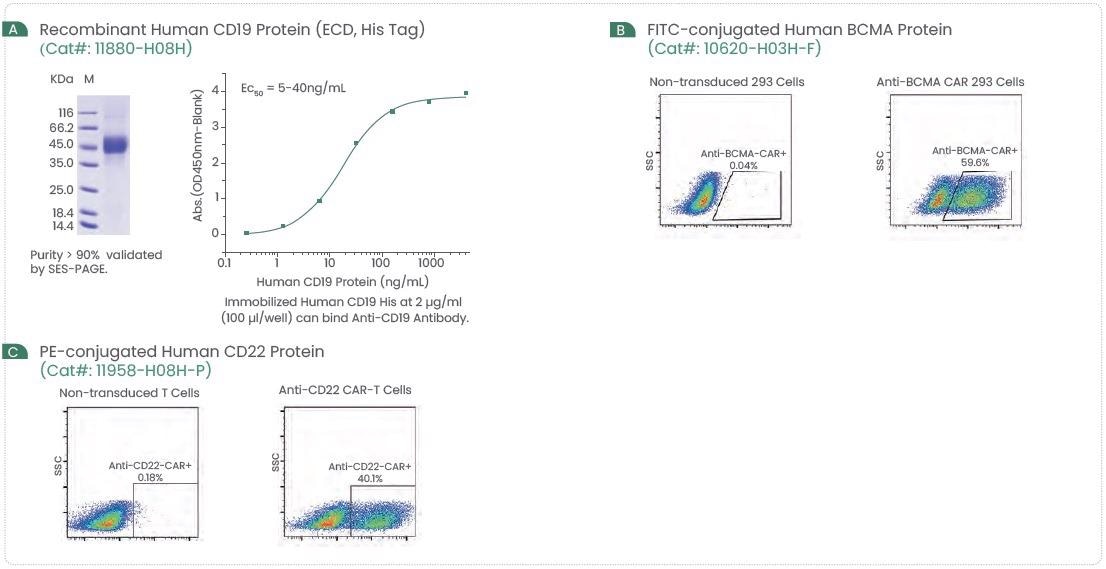
Figure 2. Examples of High-Quality CD19, BCMA, and CD22 Protein. Image Credit: Sino Biological Inc.
CAR T-cell therapy in solid tumor
Targeting solid tumors is highly complicated compared to targeting hematologic cancers, and CAR T-cells are a group of challenges that still need to be addressed. The genetic instability of tumor cells implies that they can hinder the expression of the antigens targeted by T-cells or lack the mechanism to present such antigens.
Due to the histopathological characteristics of the tumor, “trafficking” to the tumor heterogeneity, tumor site and shortage of specific antigen is insufficient.9
Solid tumors in clinical trials are increasing, such as CAR T-cells targeting mesothelin, carcinoembryonic antigen, interleukin 13 receptor alpha (IL-13Ralpha), human epidermal growth factor receptor 2 (HER2), L1 cell adhesion molecule (L1CAM), fibroblast activation protein (FAP), and epidermal growth factor receptor.
HER2, a tyrosine kinase receptor, has been overexpressed in several cancers and around 80% of Glioblastoma.10
HER2 plays a vital role in development, cell differentiation and proliferation. The HER2 gene has been linked to malignancy and a poor prognosis in several carcinomas, such as breast, prostate, ovarian, lung cancers, etc.
It has also been shown that third-generation HER2 CAR T-cells could target and kill glioblastoma cells. Efficacy was considerably enhanced when integrated with PD-1 blockade.11 Sino Biological offers extensive CAR T-cell therapy targets with high activity and purity (Figure 3).
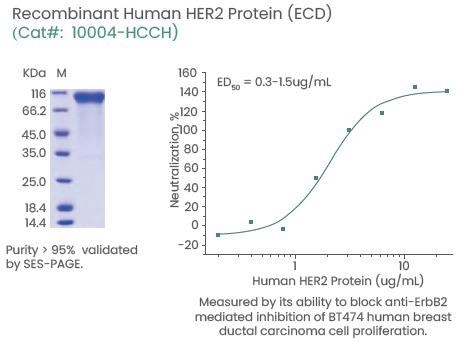
Figure 3. Examples of High-Quality HER2 Proteins. Image Credit: Sino Biological Inc.
Strategies to enhance CAR T-cell therapy
Even though CAR T-cells therapy has its benefits, their proliferation, persistence, and antitumor functions might decrease, encountering difficulties in hematologic cancer or solid tumors. Strategies are arising for developing CAR T immunotherapies.
First strategy: CAR T-cell delivery
Intravenous infusion (systemic delivery) and intracranial infusion (local delivery) could increase the security and efficiency of CAR T-cell therapy. Therefore, targeting CD19, CD20, CD30, and BCMA antigens expressed on several B-cell lymphomas has enhanced results when intravenous CAR T-cells are managed.
The successful local delivery of CAR T-cells has been also displayed in solid tumors. In glioblastoma, for instance, intracranial infusion of IL13Rα-CAR T-cells led to efficient tumor remission.12
Second strategy: Targeting multiple antigens
One of the most difficult restrictions of CAR T-cell therapy is the growth of tumor resistance to single antigen-targeted CAR constructs. Thus, numerous plans that are being developed at present depend on targeting multiple antigens to decrease the relapse rate of CAR T-cells for solid tumors and hematology cancer.
These methods utilize either dual CAR constructs or tandem CAR constructs, where a tandem CAR is known as a single CAR construct comprising two scFvs to target multiple target tumor antigens at the same time. Clinical trial results of CD19/CD22 CAR T-cell therapy have displayed efficacy in treating adult ALL and diffuse big B-cell lymphoma.
It has also been tested in preclinical models in solid tumors, such as HER2 and IL13Ra2 in Glioblastoma and HER2 and MUC1 in breast cancer. As far as these targets are concerned, dual-targeted therapy produced enhanced antitumor responses compared to single-targeted therapy.
This study demonstrates the significance of improving the selection of target antigens that enhance antitumor response and reduce antigen escape mechanisms to avoid relapse.13
Conclusion
At the beginning of this century, CAR T-cells were known as one of the most famous critical advances in cancer immunotherapy. They will be a necessary immunological technology in the 21st century, eventually defeating fatal diseases like cancer. Additional research is still required to improve CAR T-cell therapy for solid tumors.14
References
- doi: 10.1158/2159-8290.CD-12-0548
- doi: 10.15252/emmm.201607485
- doi: 10.3389/fimmu.2022.817296
- doi: 10.1186/s40364-017-0102-y
- doi: 10.1038/ni1547
- doi: 10.2478/ahp-2020-0002
- doi: 10.1016/j.critrevonc.2021.103453
- doi: 10.1038/nm.4441
- doi: 10.1016/j.smim.2015.11.003
- doi: 10.1007/s11060-007-9424-1
- doi: 10.3892/or.2019.7263
- doi: 10.1186/s13287-021-02420-8
- doi: 10.1038/s41408-021-00459-7
- doi: 10.1186/s12935-018-0685-x
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.