A virus is a simple life form made up of genetic materials (DNA or RNA) and an outer shell mostly comprised of protein. Even though viruses are considered an organism that only possesses the ‘bare minimum’ components for life, they are powerful pathogens and the cause of several major pandemics that led to a considerable loss of life and the devastation of the local and/or global economy.
In order to survive, viruses require a host in which to propagate and mutate to adapt to various environmental stresses. They interact with the host cells by binding virus surface proteins to their corresponding receptors and then fusing into the host cells.
Once they enter a host, the virus releases a load of genetic materials and are processed by the body. Virus propagation is achieved as the virus takes over various cellular machineries involved in gene transcription and protein translation (Figure 1).
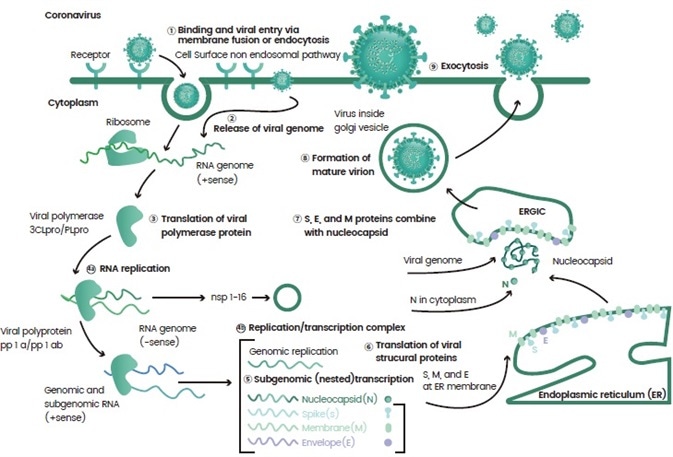
Figure 1. An example of the life cycle of a virus. Image Credit: Sino Biological Inc.
Based on their unique biochemistry, pathology and physiology, extremely specialized biochemical tools are necessary to obtain a deep insight into the pattern of interaction between a virus and its host and to uncover the mechanisms for virus propagation and assembly.
Information derived from thorough research helps pave the way for the development of effective diagnostic tools, anti-viral therapeutics and public health interventions. This article highlights the development of sophisticated biochemical tools for virology research.
These tools include antibodies, pseudovirus particles and recombinant virus proteins. It is also worth noting that, as a novel concept in vector science, particular forms of viruses are now extensively used as vehicles for gene delivery and as constituent parts in vaccines. Such applications are not detailed in this article.
Recombinant virus proteins
Proteins constitute 50% (w/w) of a virus particle, and they function in various ways. Some proteins act as the virus’s attachment and entry points (surface proteins, typically bear heavy glycosylations), while others act as a catalyst for the synthesis of the virus genome and processes of newly synthesized virus proteins (enzymes).
These virus proteins are critical to a deeper understanding of virus physiology and also advance the next generation of immunological diagnostic tools and anti-viral medications.
With recombinant protein expression technology, it is now possible to create virus proteins in significant quantities and employ them as tools across a wide range of applications in virology research.
In line with the “central dogma,” recombinant protein expression methods use a vector (plasmid) that includes the target gene encoding for the protein-of-interest for insertion into a host cell.
The target gene is either introduced into the host cell genome to guide protein translation or the protein-of-interest is generated via plasmid-mediated direct gene translation (Figure 2).
The development of various prokaryote and eukaryote host systems, completed over four decades, help meet the requisite production of recombinant proteins of key features in terms of post-translational modifications (PTMs), conformation and activities (Figure 2).
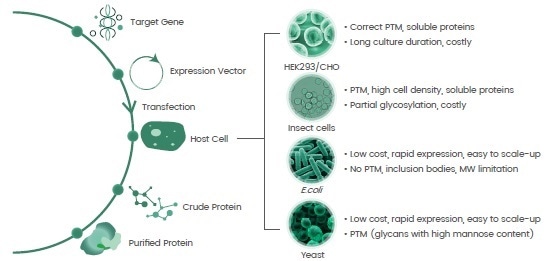
Figure 2. Basics for recombinant protein expression and host systems. Image Credit: Sino Biological Inc.
The function and biochemistry of a protein dictate the selection of expression host and strategies of purification.
For virus surface proteins, mammalian cells are primarily identified as the host-of-choice due to their complex high-order structures and extended glycosylation profiles, while a prokaryote system (e.g., E.coli) is sufficient for the production of active virus enzymes for both structural and functional studies.
On the other hand, insect cells are dynamic expression hosts and are used to create both secreted and intracellular proteins.
Proteins that the insect system expresses typically exert equivalent functionalities comparing them to those that derive from the mammalian system, but they possess a much more basic PTM profile to the advantage of structural studies.
The insect cell system acts as a very good alternative, particularly when trying to generate hard-to-express proteins.
With well-established platforms and more than a decade of experience in recombinant protein expression, Sino Biological has produced the largest collection of virus proteins anywhere in the world, with over 1000 virus proteins spanning over 100 virus strains, from the common influenza virus, coronavirus, to more fatal filoviridae such as Ebola and Marburg virus (“ProVir” Viral Antigen Bank). Some examples of virus proteins are displayed in Figure 3.
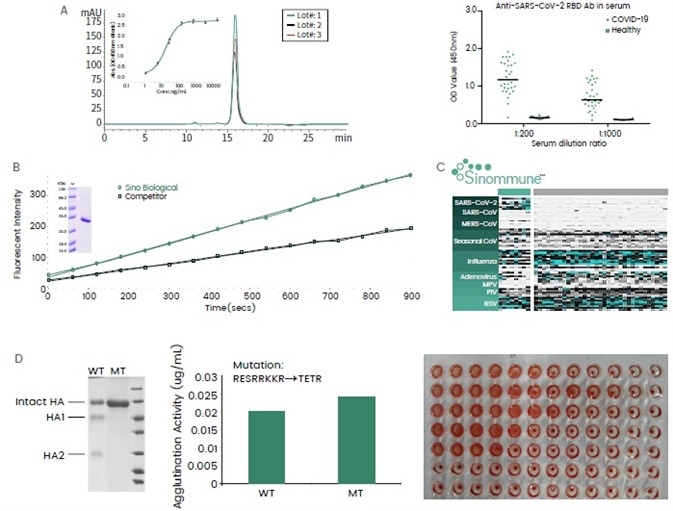
Figure 3. Examples of recombinant virus proteins from Sino Biological. (A) Receptor binding domain (RBD) of SARS-CoV-2 expressed in HEK293 cells. The protein showed good batch-to-batch consistency in terms of its purity and binding affinity against ACE2. It has been used in a serological assay to measure the serum antibody content of COVID-19 patients. (B) SARS-CoV-2 3C-like protease was expressed in insect cells with enhanced catalytic activity assayed by the cleavage of a fluorescent peptide substrate. (C) A collection of virus protein antigens for upper respiratory tract infections have been developed and formulated into the “Sinommune” chip, suitable for high-throughput and fast disease diagnostics. (D) Influenza (H5N1) hemagglutinin (HA) protein expressed in insect cells with agglutination activities. Mutations at the furin cleavage site abolished protein cleavage by host cells without compromising the agglutination activities. Image Credit: Sino Biological Inc.
As an extension of the traditional protein expression methods, the transient protein expression method by HEK293 facilitates the creation of a high-throughput protein expression platform where hundreds of constructs can be simultaneously expressed to produce a recombinant protein library.
This technical platform is particularly useful when trying to deal with the high-frequency mutation rate of RNA viruses.
So far, this service platform has been utilized to produce over 100 influenza proteins and over 80 SARS-CoV-2 RBD variants to assist high-throughput screening campaigns to determine broad-spectrum neutralizing antibodies.
Virus-specific antibodies and pseudovirus
When facing a novel pathogen, the host will stage a series of immune responses to eradicate the threat. During this process, pieces of the pathogen are identified by immune cells, followed by the creation of a pool of antibodies (immunoglobulin) with the specific task of “flagging” the pathogen for elimination by various effector cells.
Virus (pathogen) specific antibodies with high specificities and sensitivities are practical tools for virus identification in a serological assay and neutralization if employed as a therapeutic agent.
Equipped with extensive antibody production platforms as well as a library of comprehensive pre-existing antibodies, Sino Biological is vastly experienced in virus-specific antibody discoveries.
During the ongoing COVID-19 pandemic, efforts were launched in pre-existing antibody library screenings and new antibody discoveries via animal immunizations.
Over 30 different antibodies against the SARS-CoV-2 spike or nucleocapsid (NP) protein have been acquired, among which a few antibody pairs were detected to formulate ELISA assays with pg/mL sensitivities (Figure 4A).
The NP assays have demonstrated the capacity to be mutation-proof when tested against NP mutants from the B1.1.7 lineage, with only a small decrease of sensitivities witnesses (Figure 4C).
Finally, ultra-sensitive NP antibodies have also been determined throughout the screening process to enhance the detection limit of NP antigen at a phantom molar level in the Simoa HD-X system (Figure 4B).
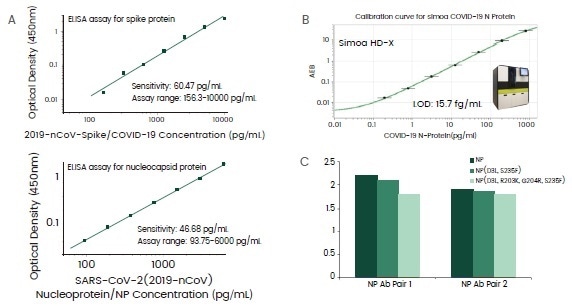
Figure 4. Highly sensitive antibody pairs for serologic assay development. Image Credit: Sino Biological Inc.
Most RNA viruses demonstrate high-frequency mutagenesis. Viruses evolve through a series of randomized mutations to adjust to the new immune landscape. The mutations could also have grave implications when occurring at key epitopes and thus significantly compromising antibody efficacy.
The production of a mutant protein library is practical for assessing the impact of mutation on antibody efficacy, but the variations in binding affinity do not directly correlate to the overall virus behavior.
However, pseudovirus is a practical tool that makes up for such shortcomings and boosts the biological relevance in the assessments of the biology of mutations.
Making good use of current viral vector manipulation techniques, the protein-of-interest can be engineered onto the surface of the virus particles while a reporter gene is encapsulated and activated once the pseudovirus is introduced into the host cells.
The readouts of such assays are typically conducted in the form of fluorescent intensities, and the results offer useful information to assert the virulence of a virus and support the evaluation of the impact of mutations. One such example is shown in Figure 5.
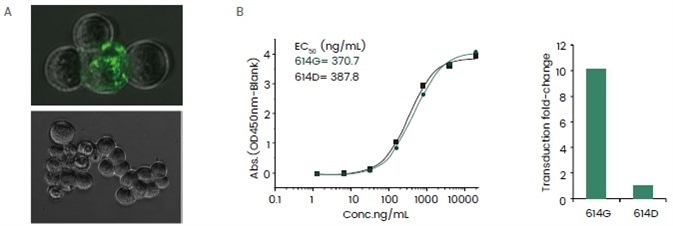
Figure 5. Application of SARS-CoV-2 pseudovirus in assessing the impact of D614G mutation. (A) A proof of concept experiment showed that the SARS-CoV-2 pseudovirus is able to bind and internalize into the host cell. Top: SARS-CoV-2 pseudovirus infected ACE2 over-expression HEK293 cell line; bottom: negative control. (B) The D614G mutation did not cause a significant alteration in ACE2 protein binding affinity judging by ELSA (top) while a SARS-CoV-2 pseudovirus containing such mutation resulted in a ~5-fold increase in the transduction fold-change, indicating a higher infectious potency given by the D614G mutation. Image Credit: Sino Biological Inc.
Conclusion remarks
With the near-constant growth of industrialization and expansion of the human habitat, zoonotic viruses are lying in wait, ready to emerge, and pandemics are part of the imminent future.
Recombinant virus protein, virus-specific antibodies and pseudovirus are excellent tools that help scientists understand virus biology and support the efforts of pandemic control.
However, for effective pandemic prevention, rational planning of urbanization is crucial to establish optimized processes of resource distribution and the use of new, alternative technologies to achieve a long-term and continuous development of human society.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.