Biological samples used for alcohol analysis are usually preserved using sodium fluoride. Before determining the alcohol content of all the submitted samples that include samples obtained from vehicle drivers who are alleged to be driving under the influence of alcohol by gas chromatography, the samples need to be tested for adequate preservation.
Sufficient sample preservation is critical in sample analysis because samples that are inadequately preserved experience the growth of microorganisms and/or glycolysis, which in turn, results in ethanol production.
An ion meter, a fluoride-selective electrode (FISE), and certified NaF standards have been used in the past to perform direct potentiometric measurements for determining the sodium fluoride level. Here, the level of sodium fluoride is determined by manually immersing the electrode into the blood sample and recording the results.
Two distinct automated analysis methods that facilitate reduction of the time consumed and complexity of the process are discussed in this article. The first method involves direct potentiometric measurement of the fluoride content in a blood sample after adding TISAB and deionized water. In the second method, the sample aliquot consisting of La(NO3)3 is titrated subsequent to the addition of a buffer solution.
Instrumentation
Figure 1 shows the instrumentation setup used for determining the fluoride content present in a blood sample automatically. The system comprises an 855 Robotic Titrosampler featuring three 800 Dosinos and a PC, controlled by the tiamo™ software package.

Figure 1. Instrumentation setup for the automatic determination of fluoride content in the blood sample
Using the 855 Robotic Titrosampler equipped with three 800 Dosino, the NaF standards and/or blood samples are pipetted out, buffer or TISAB solutions are added, and titration is performed with La(NO3)3.
The FISE and the reference electrode, PFA titration vessel, and magnetic stirrer are coupled to the right side-fitted micro-titration vessel lid. Additionally, rinsing nozzles, dosing tips, and an aspiration tip to aspirate the spent sample solution into waste are also provided in the vessel lid. The 849 Level Control fitted to the rinsing and waste containers prevents overflow of waste containers and/or drying up of pumps by continuously observing the waste and rinsing levels.
There are two bespoke sample racks specially manufactured for fluoride analysis. Two automated systems that use two different racks are used in each of these three different labs. One rack consists of 322 places in total and is used for glass vials, and the second rack has 107 places in total to hold special McCartney bottles.
There are two other cap-holding racks specially designed for each of these two systems. During analysis, caps are stored by these racks because samples are obtained either from the McCartney bottles or from the opened glass vials. Cap holding racks were needed because each opened via should be closed only with the original cap after analysis to ensure integrity of the sample.
Analytical Procedure
Samples obtained from drivers alleged with drunken driving are taken in glass sample vials, while post-mortem blood samples obtained from different mortuaries for verifying insurance claims for alcohol-related deaths are taken in the McCartney sample bottles. An LIMS is used to log the sample batches, and tiamo™ receives sample data wirelessly through sample tables.
The designated sample rack is stacked with the blood samples for testing as per the relevant sample table. Further processes like sample transfer, titration or direct potentiometric measurement, result calculation, rinsing and cleaning are completely automated. Before exporting the results to LIMS, they are manually verified.
Pipetting of Standards and Blood Samples
The first step is filling the entire transfer tube with the buffer solution of pH 6, followed by aspirating a fixed sample volume of 1.5mL aliquot for both standards and samples, lodged between two air gaps, from the sample vessel.
The pipetting equipment consisting of the 800 Dosino with 10mL dosing unit and a 10mL transfer tube with pipette tip is then used to transfer the sample into the external titration vessel. The final step is rinsing the transfer tube using 10mL buffer solution into the titration vessel with the addition of 10mL ultrapure water.
Calibration
The PP vials are filled with three certified NaF standards (0.8%, 1.5%, and 3.0%), and then located at three specifically designated positions on the sample rack. True values corresponding to these standards are designated as common variables STD 1 to 3. A three-point calibration curve is recorded based on the certified NaF standards, starting from 0.8%, as the first step of the sample series (Figure 2).
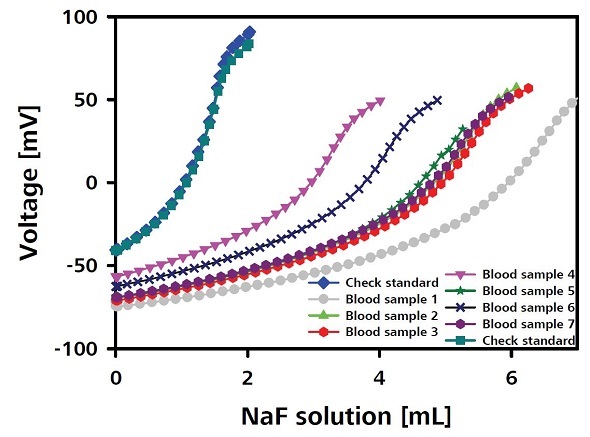
Figure 2. Three point calibration curve for the sample series.
Under continuous stirring conditions, the potential is determined and the mV value is noted. The sensors, buret tips, tubing, magnetic stirring bar, and the vessel are then cleaned meticulously. The entire process is repeated for the standards 2 and 3, i.e., 1.5% and 3.0% NaF, respectively, followed by recording the corresponding three-point calibration curves automatically. Verification of the accuracy of measurements is done after every 10 samples using a certified check standard of 1.0% NaF content. Determination of the fluoride content in blood samples is also carried out in the same order. Table 1 shows the fluoride content for various blood samples.
Table 1. Fluoride content in various blood samples and standards
|
Sample
|
Fluoride content
(%)
|
|
Check standard (1% fluoride)
|
1.01
|
|
Blood sample 1
|
3.61
|
|
Blood sample 2
|
2.85
|
|
Blood sample 3
|
3.30
|
|
Blood sample 4
|
2.27
|
|
Blood sample 5
|
3.74
|
|
Blood sample 6
|
3.68
|
|
Blood sample 7
|
4.51
|
|
Check standard (1.0% fluoride)
|
1.02
|
|
Check standard (0.8% fluoride)
|
0.79
|
|
Check standard (1.5% fluoride)
|
1.53
|
|
Check standard (3.0% fluoride)
|
3.03
|
The automated system is capable of analyzing as many as 317 samples in a single continuous run with the help of the special sample rack holding the glass vials. Analysis of each blood sample is completed in 3 minutes, which includes the time for transfer and rinsing. The specially designed rack housing McCartney bottles enables the analysis of 102 samples in a single run.
Fully Automated Titration
The process involves filling the transfer tube with a buffer solution of pH 6 and creating an air gap to aspirate a fixed volume of 1.5mL aliquot of the sample from the sample vessel creating another air gap. Subsequently, another air gap is created. The pipetting set up consisting of 800 Dosino 10mL dosing unit and a 10mL transfer tube with pipette tip is used for transferring the sample into the external titration vessel.
This is followed by rinsing the transfer tube using a 10mL buffer solution into the titration vessel, and the addition of 10mL deionized water. Titration of the sample is done using c(La(NO3)3 equal to 0.1mol/L. A Gran Plot is used to evaluate the endpoint. Rinsing and emptying the titration vessel are done for multiple times before starting the subsequent analysis. Figure 3 shows the fluoride concentration plot.
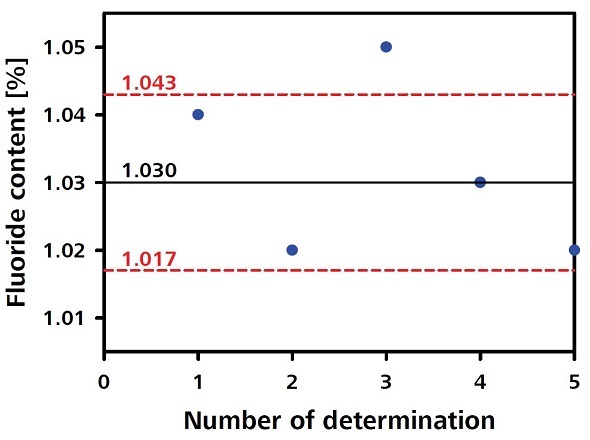
Figure 3. Fluoride content plot
The titer determination of the c(La(NO3)3 equal to 0.1 mol/L with a certified standard 3.0% NaF is carried out in the same order. Table 2 shows the titer determination values for check standards.
Table 2. Titer determination values for fluorine
|
Check standard (1% fluoride)
|
Fluoride content (%)
|
|
Determination 1
|
1.04
|
|
Determination 2
|
1.02
|
|
Determination 3
|
1.05
|
|
Determination 4
|
1.03
|
|
Determination 5
|
1.02
|
|
Mean
|
1.03
|
|
RSD
|
1.3%
|
Conclusion
Globally, several road accidents are caused by drunken driving. The majority of the countries do not allow driving of vehicles when the permitted levels of blood alcohol content (BAC) are exceeded. Intoxication is defined by BAC, which can be measured objectively. Due to the influence of enzymes and bacteria, blood sugar may be fermented into ethanol, resulting in an increase of BAC.
However, the formation of alcohol is reduced when a known quantity of an enzyme inhibitor like fluoride is added. This is the reason some countries demand the reporting of fluoride content of the sample along with the BAC value.
The above experiments have demonstrated that complete automation of fluoride determination in blood samples can be successfully performed by precipitation titration with La(NO3)3, or with standard addition at lower concentrations. The presented setup provides more precise results owing to the constant pipetting and measurement protocols.
References
- Metrohm Application Bulletin AB-082, Determination of fluoride with the ion-selective electrode, http://products.metrohm.com (search for AB-082).
Acknowledgements
Produced from materials authored by H. Risse1 and J. Minnaar2 from:
1Metrohm AG, CH-9101 Herisau/Switzerland.
2Metrohm South Africa, Gallo Mannor 2052/South Africa.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.