Diseases are diagnosed, treated and prevented with nuclear medicine using radioactive substances called radiopharmaceuticals. These substances contain a radionuclide, which is a radioactive isotope attached to a biologically active or inert molecule. Due to the presence of excess protons or neutrons, these unstable isotopes undergo radioactive decay, thus releasing subatomic particles or gamma rays.
The proton (p+) transforms into a neutron in proton-rich nuclides, resulting in the emission of a positron (β+ particle) along with a neutrino (ν) as per the equation below:
(p+) -> (n) + (β+) + (ν)
There is a loss in the kinetic energy of the positron while traversing through surrounding media. The positron is then coupled to an electron, and as a result, the positron and electron are annihilated and their masses are directly transformed into energy. Annihilation radiation results in the emission of two photons (gamma rays) into opposite directions, each with 0.511MeV energy.
me+ + me– -> 2γ
Such photon pairs can be detected by advanced scanners through coincidence detection. Calculation of 3D images of tissue structures is done using the collected data. Trace is another term for the positron-emitting radionuclide.
Some of the short-term, cyclotron-created radionuclides commonly used in radiopharmacy are 11C, 15O, 18F and 13N, with half-lives of 20.38, 2.03, 109.7 and 9.96 minutes, respectively. Figure 1 shows the working principle of PET.
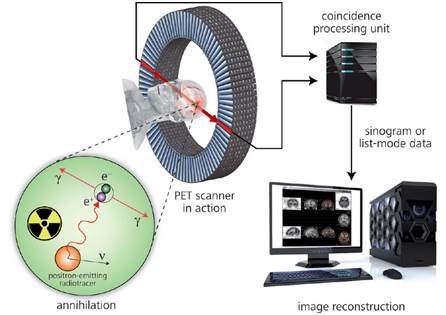
Figure 1. Principle of positron emission tomography
Radiopharmaceuticals
The radionuclide is injected into a living animal or human by combining it with biologically active or inert molecules like blood flow tracers [15O] water or [15O] butanol that are absorbed by a specific organ under study. The patient is placed under the PET scanner after the tissue of interest is injected with an adequate quantity of the radiopharmaceutical.
The generated photons are tracked and 3D images of their sources are produced by computers equipped with advanced software. These images facilitate molecular level investigations of the biochemical, physiological and pharmacological functions. Such investigations help in the early detection of health disorders like cardiovascular disease, cancer and even neurological disorders, much before the symptoms start showing up.
Production of PET Radiopharmaceuticals
A cyclotron (Figure 2) is used for the synthesis of radionuclides like 11C, 13N, 15O, and 18F, which are used in PET experiments. Radionuclides are produced by the irradiation of a prepared target with a beam of accelerated charged particles. The radionuclides are then isolated and artificially integrated into a radiotracer.
Due to the similarity in the size of the fluorine and hydrogen, fluorine atom is suitable for replacing the hydrogen atoms present in organic molecules because fluorine acts like pseudohydrogen.

Figure 2. One of the two ITP cyclotrons for the production of PET radionuclides.
Fluorine (18F) is a positron-emitting radionuclide that is generated when an 18O-enriched water target is subjected to proton bombardment. 18F finds many important applications in diagnostic nuclear medicine.
The 18O(p, n)18F reaction is brought about by highly accelerated protons reacting with the atomic nucleus of 18O, resulting in the emission of a neutron (n) and 18F. This is followed by the instant decay of 18F with a half-life of 109.7 minutes due to positron emission, generating a stable isotope 18O.
The radionuclide 18F is then isolated from the target water and added to the chemical compound used in radiosynthesis. The 18F radiotracer obtained subsequent to the isolation and purification is then subjected to a series of QC tests before it is injected into a patient.
[18F] Fluorodeoxyglucose
The substitution of the hydroxyl group present at the 2’ position of the glucose molecule by [18F] fluorine results in the formation of a glucose analog called [18F] fluorodeoxyglucose or [18F] FDG (Figure 3). This compound is used for examining the application and metabolism of glucose in the brain, heart and lungs.
In oncology, [18F] FDG is also used for characterizing various types of tumors by studying the abnormality in their glucose metabolism. After administration, the mode of transport of [18F] FDG for incorporation into the cells is the same as glucose. However, [18F] FDG remains unchanged in the cells because it is not metabolized by the cells like glucose.
This unchanged state of [18F] FDG facilitates PET tomographic imaging. The two main advantages of [18F] FDG are its comparative half-life of [18F] of roughly 2 hours, which facilitates transport to sites that lack a cyclotron, and a wide choice of labeling procedures. These are the key reasons for its widespread adoption in PET diagnosis.
[18F] Fluorocholine
Phospholipids are biosynthesized in cells using choline as a precursor. They are the important constituents of the cell membrane. There is an increased metabolism of membrane components along with an increased choline uptake at a tumor site.
Therefore, radiolabeled choline tracers are more suitable for cancer detection. A recent addition to the list of PET radiotracers is [18F] fluorocholine, which enables in vivo imaging of choline metabolism.
The tumor-detecting radiotracer [11C] choline forms the basis for these PET radiotracers. The long half-life is the driving force of the 18F-labeled derivative production, allowing the supply of this tracer to PET institutions that do not have a cyclotron at their sites.
![Chemical structures of two PET radiopharmaceuticals. (a) In [18F] FDG, the hydroxyl group at the 2‘ position of normal glucose is substituted by 18F. (b) In [18F] fluorocholine, a [18F] fluoroalkyl group is attached to the nitrogen atom of N,N-dimethylaminoethanol (DMAE).](https://www.news-medical.net/image-handler/picture/clip_image006_0004_2.jpg)
Figure 3. Chemical structures of two PET radiopharmaceuticals. (a) In [18F] FDG, the hydroxyl group at the 2‘ position of normal glucose is substituted by 18F. (b) In [18F] fluorocholine, a [18F] fluoroalkyl group is attached to the nitrogen atom of N,N-dimethylaminoethanol (DMAE).
Metrohm`s Radio Ion Chromatography
The QC control labs of the PET Technological Institute (ITP) based in Madrid are equipped with two different ion chromatography systems from Metrohm (Figure 4). The first ion chromatography system, installed in 2010, was customized for the QC of the radionuclide [18F] fluoride and its derivative radiotracers, namely [18F] FDG and [18F] fluorocholine.
High throughput and accurate and reproducible analytical results are the key requirements for this process. The QC of all the three production lines is managed by the same multichannel radio IC.

Figure 4. Dr. Jesús Chesa-Jiménez, quality control department supervisor at ITP using Metrohm‘s ion chromatography system.
The chromatograms for the radioactivity and the conductivity of [18F] fluoride produced by the cyclotron are shown in Figure 5. Conversion of radiation units and counts per second (cps) to mV is done by the IC software. Table 1 lists the corresponding chromatographic conditions.
![(a) Conductivity and (b) radioactivity chromatogram of cyclotron-produced [18F] fluoride. In the subsequent radiosynthesis (nucleophilic fluorination), trace (i.e., very low) quantities of [18F] fluoride ions are used to form carbon-fluorine bonds.](https://www.news-medical.net/image-handler/picture/clip_image010_7.jpg)
Figure 5. (a) Conductivity and (b) radioactivity chromatogram of cyclotron-produced [18F] fluoride. In the subsequent radiosynthesis (nucleophilic fluorination), trace (i.e., very low) quantities of [18F] fluoride ions are used to form carbon-fluorine bonds.
Table 1. Chromatographic conditions for the quality control of [18F] fluoride, [18F] FDG, and [18F] fluorocholine
|
|
[18F] fluoride
|
[18F] FDG
|
[18F] fluorocholine
|
|
Column
|
Metrohm A Supp 5 - 150/4.0
|
Metrosep Carb 1 - 150/4.0
|
Metrosep C 4 - 150/4.0
|
|
Column temperature
|
45°C
|
25°C
|
40°C
|
|
Sample volume
|
10μL
|
10μL
|
10μL
|
|
Eluent
|
3.2mmol/L sodium carbonate 1.0mmol/L sodium hydrogen carbonate
|
0.1mol/L sodium hydroxide
|
1.7mmol/L nitric acid 0.7mmol/L dipicolinic acid
|
|
Flow rate
|
0.7mL/min
|
1.0mL/min
|
1.5mL/min
|
|
Detection
|
Conductivity detection after chemical suppression
|
Pulsed amperometric detection
|
Conductivity detection
|
|
Analysis time
|
8 minutes
|
18 minutes
|
14 minutes
|
Advantages of Radio Ion Chromatography Systems at ITP
At the ITP, three QC systems are integrated into the single ion chromatography system for PET pharmaceuticals. Automatic direction of the flow to all three channels can be executed by the same injection system.
Determination of [18F] FDG, [18F] fluoride and [18F] fluorocholine can be done separately by choosing from a wide choice of columns, mobile phases and detectors for each of them. The MagIC Net™ software manages the entire system operation and data acquisition.
The system is fitted with the Dosino technology from Metrohm for automated sample injection. This technology enables the accurate and precise aspiration of extremely low sample volumes. Complete automation of tasks such as liquid handling, rinsing and dosing by the MagIC Net software prevents any carryover. The Metrohm IC system’s modular design ensures the safety of operators by allowing for the deployment of the necessary lead shielding.
The use of the adequately thick lead shielding lowers the radiation from the radiotracers placed in the separation columns to a safe level, and the 5-cm-thick bespoke lead housing accommodates the injection valve. Further, users are protected from gamma radiation exposure owing to the use of a lead sample holder.
Due to their unique properties, special tests need to be performed for radiopharmaceuticals before injecting the radiotracer into the patient. The radiochemical and the chemical purity of the radiotracer need to be checked during QC.
The ratio between the bound ([18F] FDG) and unbound ([18F] fluoride) forms of a radionuclide is defined as the radiotracer’s radiochemical purity. The quality of the PET scan image and the protection of the patient from unnecessary exposure to radiation are based on the radiochemical purity value. The first step is the determination of the concentration of the radionuclide produced by the cyclotron.
The next step is determining the chemical purity and the concentration of radiopharmaceutical derived from the radionuclide. These steps help quantify the excessive precursors and impurities derived from radiolabeling. The analysis time is below 10 minutes. The results are presented in Figures 6 and 7.
![Chromatograms for the radiopharmaceutical [18F] FDG.](https://www.news-medical.net/image-handler/picture/clip_image012_6.jpg)
Figure 6. Chromatograms for the radiopharmaceutical [18F] FDG. (a) IC-PAD chromatogram with the glucose precursor, the carrier-free [18F] FDG, and the impurity chlorodeoxyglucose. (b) Radioactivity chromatogram of the [18F] FDG. The IC software converts the radiation units, counts per second (cps), to mV. Chromatographic conditions are shown in the table.
The peaks of the glucose precursor, the chlorodeoxyglucose impurity, and the carrier-free [18F] FDG are depicted in Figure 6. High levels of residual DMAE result from this labeling reaction (Figure 7). Determination of other byproducts like bromocholine from this reaction is also possible. The conversion of the radiation units from counts per second to mV is done by the IC software.
The chromatogram presented in Figure 7 for the reaction mixture not only reveals the [18F] fluorocholine radiotracer in nanomole quantities but also shows the presence of calcium impurities in trace levels and N,N-dimethylaminoethanol (reactant) in considerable quantities. Table 1 summarizes the corresponding chromatographic conditions.
![Chromatograms for the radiopharmaceutical [18F] fluorocholine.](https://www.news-medical.net/image-handler/picture/clip_image014_5.jpg)
Figure 7. Chromatograms for the radiopharmaceutical [18F] fluorocholine. (a) Conductivity and (b) radioactivity chromatogram of the [18F] fluorocholine reaction mixture. [18F] Fluorocholine is synthesized by 18F-fluoroalkylation of N,N-dimethylaminoethanol (DMAE) using gaseous 18F-fluorobromomethane.
Due to the short half-lives of positron-emitting radiopharmaceuticals, there is a need for conducting quick QC. The determination process and the rinsing steps of the sample injection circuit and the detector pathways are computer controlled and optimally synchronized, thus ensuring quick and accurate analyses (Figure 8).

Figure 8. The IC specialists of Gomensoro adapted the Metrohm IC system to the needs of ITP (left to right): Javier Espuelas (Applications Laboratory Manager), Cristobal Hidalgo (Calibration Manager), Vicente Ubeda (Sales Manager), Juan Lopez (Metrohm Sales Manager), Miguel Espinosa (Ion Chromatography Product Manager), Andoni Epalza (IC Application Specialist), and Tomas Sanz (IC Application Specialist).
Conclusion
The stringent requirements of the pharmacopoeial regulations and radiopharmaceutical industry are adequately met by the highly customizable chromatography system from Metrohm. QC requirements of different production lines are managed by a single multichannel radio IC. The Metrohm IC not only delivers high quality results, but also guarantees excellent ruggedness, user safety and low cost of maintenance.
References
- D. Slaets, S. De Bruyne, C. Dumolyn, L. Moerman, K. Mertens, and F. De Vos, Reduced dimethylaminoethanol in [18F] fluoromethylcholine: an important step towards enhanced tumour visualization, Europ J Nucl Med Mol Imaging 37(11), 2136–2145 (2010).
- Cyclotron-produced radionuclides: physical characteristics and production methods, Technical Report Series No. 468, International Atomic Energy Agency, Vienna, 266 p. (2009).
- D. Kryza, V. Tadino, M. Azuzurra Filannino, G. Villeret, and L. Lemoucheux, Fully automated [18F] fluorocholine synthesis in the TracerLab MXFDG Coincidence synthesizer, Nucl Med Biol 35, 255–260 (2008).
- J. Passchier, Fast high-performance liquid chromatography in PET quality control and metabolite analysis, Q J Nucl Med Mol Imaging 53, 411–416 (2009).
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.