The Rancimat technique is an accelerated aging test. Air is passing through the sample in the reaction vessel at a constant high temperature and fatty acids are oxidized in this process. Volatile, secondary reaction products are formed at the end of the test, which are carried into the measuring vessel via the air stream and absorbed in the measuring solution (deionized water).
Due to the absorption of the reaction products, the continuously recorded electrical conductivity of the measuring solution grows. Hence, their appearance can be detected. The time until secondary reaction products are detected is known as induction time. It characterizes the oxidation stability of fats and oils. This article supplies a detailed description of the requisite sample preparation steps.
Samples
Oils, fats, oil- and fat-containing products
Instruments
| . |
. |
| 892 Professional Rancimat |
2.892.0010 |
| Set for the determination of the temperature correction |
6.5616.100 |
| Auxiliary instruments for sample preparation |
|
| Laboratory balance (resolution ± 0.01 g) |
|
Reagents
For Sample Preparation
Petroleum ether, low boiling, boiling point 30 - 40 °C, puriss. p.a., CAS 101216-46-5 Sample preparation.
Fat-containing solids – Cold extraction
Before determination, fat from samples that possess a complex matrix, e.g. foodstuffs like powdered milk, biscuits, mayonnaise, chocolate, etc. must be extracted. Ideally, since heating would alter the fat, this is performed using cold extraction. The sample must be crushed before the extraction if it is not already powdered or liquid.
Enough sample to extract approx. 10 g fat (enough for two measurements) is weighed into a conical flask. Around three times the sample volume of low boiling petroleum ether is added. The extraction is carried out by stirring for an hour minimum.
The petroleum ether phase is then separated from the residues by either filtering in case of solid samples or by a separating funnel in case of liquid samples and transferred into a round bottom flask. The petroleum ether is distilled off at 20 - 30 °C under vacuum, e.g., with a rotary evaporator.
Fat-containing Solids – Direct Measurement
Solids with high volumes of fat, like nuts and oil seeds (e.g., sunflower seeds, hazelnuts, almonds, sesame seeds, etc.), can be measured directly. Before the sample is weighed in, it must be crushed and homogenized, e.g., by a mortar. Care should be taken that the sample is not contaminated by traces of transition metals and not overheated.
Solid Fats
Solid fats as a group of animal or vegetable fats which are solid at room temperature and melt at elevated temperature can be measured directly. The sample can be melted on a water bath in the instance of problems weighing the sample into the bottom part of the reaction vessel. Care has to be taken that the water bath temperature is not higher than the melting point of the sample or deterioration of the sample can be expected.
Water-containing Fats
Water-containing fats (butter, margarine) can also be weighed indirectly. In order to compensate for the sample loss caused by evaporation of the containing water, the sample size must be increased.
Liquid Oils
Liquid oils as a group of animal or vegetable fats which are liquid at room temperature may be measured directly. In order to weigh the sample directly into the reaction vessel, a disposable plastic Pasteur pipette is employed.
Analysis
Preparation of the Rancimat
The heating block is heated up to the respective temperature.
Preparation of the Measuring Vessel
Together with the measuring vessel cover, the measuring vessel is filled with 60 mL deionized water and put on the Rancimat. It is advisable to increase the volume to compensate for evaporation loss for longer analysis times (more than 72 hours). An evaporation rate of 5 - 10 mL water per day must be considered. The electrode must be immersed into the measuring solution at any time.
Preparation of the Reaction Vessel
A new reaction vessel is utilized for each determination. The reaction vessel is air-cleaned inside and outside by a sharp stream of nitrogen in order to remove particles (e.g. from the cardboard box). Then the sample is weighed straight into the reaction vessel.
For samples that melt at elevated temperatures and for liquid samples, a sample size of 3.0 ± 0.1 g is utilized. For samples with considerable water content (more than 5%), the sample size has to be larger to compensate for the decrease in volume when the water evaporates. The air inlet tube must always be immersed in the sample.
Solid samples that do not melt must only cover the bottom of the reaction vessel. In this instance, 0.5 - 1 g of the powdered sample is weighed into the reaction vessel. The reaction vessel is closed with a reaction vessel cover assembled with an air inlet tube.
Determination
The temperature of the heating block must be stable before the determination begins. The two tubings between reaction vessel and Rancimat, and between measuring vessel and reaction vessel are connected. Then the reaction vessel is put in the heating block and the measurement begins immediately.
Parameters
| . |
. |
| Sample size |
Liquid samples: 3.0 ± 0.1 g
Solid samples: 0.5 … 1 g |
| Measuring solution |
60 mL |
| Temperature |
80 … 160 °C |
| Gas flow |
20 L/h |
| Evaluation |
Induction time |
| Evaluation sensitivity |
1.0 |
The measuring temperature is dependent on the oxidation stability of the sample. Typical temperatures of between 80 and 160 °C are appropriate for the sample varieties outlined in this article, 50 to 220 °C are viable. Typically, the majority of tests are performed at 120 °C (Iower stability – lower temperature). The general rule is: the induction time is lowered by a factor of two for a temperature increase of 10 °C.
Typical Results
Vegetable Oils and Fats
| Sample |
Temperature/°C |
Induction time/h |
| Canola oil |
130 |
12 … 17 |
| Canola oil, hydrogenated |
140 |
10 … 11 |
| Citrus oil |
90 |
approx. 0.5 |
| Cocoa butter |
120 |
9 … 15 |
| Coconut oil |
120 |
approx. 33 |
| Coffee oil |
110 |
approx. 0.25 |
| Corn oil |
120 |
approx. 5 |
| Cottonseed oil |
120 |
2 … 3 |
| Hazelnut fat |
120 |
10 … 12 |
| Hazelnut oil |
120 |
7 … 11 |
| Linseed oil |
110 |
0.5 … 2 |
| Margarine |
120 |
2 …. 6 |
| Olive oil |
120 |
6 … 11 |
| Orange oil |
90 |
approx. 2 |
| Palm oil |
120 |
7 … 12 |
| Peanut fat |
120 |
9 … 10 |
| Peanut oil |
120 |
3 … 15 |
| Pumpkin seed oil |
120 |
approx. 7 |
| Rapeseed oil |
120 |
3 … 5 |
| Safflower oil |
120 |
1 … 2 |
| Sesame oil |
120 |
approx. 5 |
| Soybean oil |
120 |
1 … 7 |
| Sunflower oil |
120 |
1 … 4 |
| Sweet almond oil |
120 |
approx. 4 |
| Walnut oil |
120 |
approx. 2 |
Animal Oils and Fats, Direct Determination
| Sample |
Temperature/°C |
Induction time/h |
| Butter |
120 |
3 … 6 |
| Chicken fat |
110 |
approx. 0.5 |
| Fish oil |
80 |
approx. 0.25 |
| Kidney fat |
110 |
3 … 4 |
| Lard |
100 |
1 … 3 |
| Pigeon fat |
110 |
approx. 0.3 |
| Tallow |
120 |
3 … 8 |
Solid Samples, Direct Determination
| Sample |
Temperature/°C |
Induction time/h |
| Butter cookies |
160 |
approx. 6 |
| Coconut flakes |
160 |
approx. 17 |
| Hazelnuts |
120 |
approx. 22 |
| Instant noodles |
120 |
15 … 30 |
| Peanuts |
110 |
approx. 10 |
| Potato chips (crackers) |
140 |
approx. 10 |
Solid Samples After Extraction
| Sample |
Temperature/°C |
Induction time/h |
| Baby food |
120 |
1 ….2 |
| Hazelnuts |
120 |
7 ….13 |
| Mayonnaise |
120 |
1 ….4 |
| Peanuts |
120 |
1 ….2 |
| Potato chips (crackers) |
140 |
approx. 2 |
| Powdered milk |
120 |
4 ….32 |
| Salad dressing |
120 |
approx. 2 |
Examples
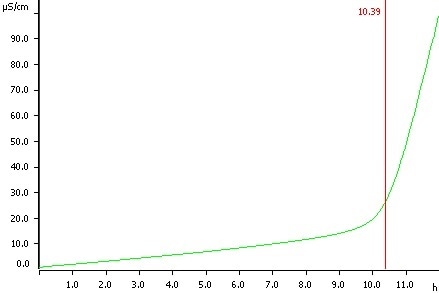
Olive oil, temperature 120 °C, induction time 10.39 h
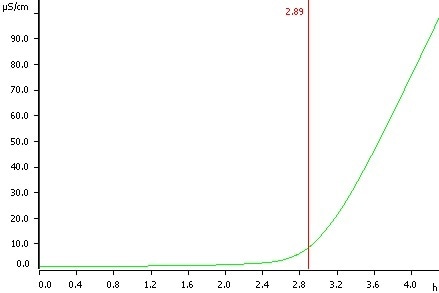
Sunflower oil, temperature 120 °C, induction time 2.89 h
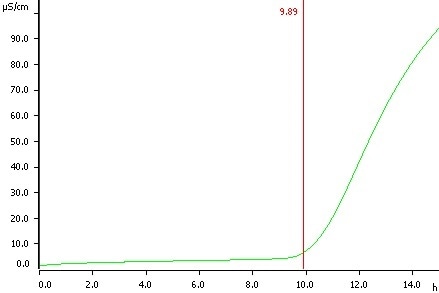
Peanuts (direct determination), temperature 110 °C, induction time 9.89 h
Comments
Any type of particles, contamination, or scratches in the glass can catalyze reactions and influence the result. Contaminations can reduce the reproducibility of the results or lead to incorrect results. So, it is recommended to utilize a new air tube and reaction vessel for each experiment and blow off particles using a sharp stream of nitrogen.
In the calculation of the induction time, the temperature is the most important factor. It is crucial to establish the ‘temperature correction’ value correctly, particularly if results from different instruments are to be compared.
For the application to ensure a sufficient supply of oxygen for the oxidation of fatty acids and a reliable transfer of the reaction products from the reaction vessel to the measuring vessel, the gas flow is key. In addition, it stirs the sample and guarantees a homogeneous temperature in the sample.
There is no influence of the gas flow on the result beyond that, if the cooling effect is compensated by the proper adjustment of the ‘temperature correction’. For samples that melt at elevated temperature and liquid samples, the sample size is not a critical parameter. The lower limit for the volume is set by the air tube which must be immersed in the sample.
The maximum volume to be utilized is around 12 mL, a proper heating of the sample cannot be guaranteed for anything exceeding that. For the direct determination of solid samples, the sample size can be a crucial parameter. No homogeneous temperature can be guaranteed in a larger volume since the air stream is unable to mix the sample. Thus, a small sample size that only covers the bottom of the reaction vessel should be used.
References
- StabNet, Tutorial
- 892 Professional Rancimat, Manual
- Läubli M. W., Bruttel P. A.: Determination of the oxidative stability of fats and oils: Comparison between the active oxygen method (AOCS Cd 12-57) and the Rancimat method, JAOCS 63 (1986) 792-795.
- Läubli M. W., Bruttel P. A., Schalch E.: A modern method of determining the oxidative stability of fats and oils, Int. Food Marketing & Technology 1 (1988) 16-18.
- Warner K., Frankel E.N., Mounts T.L.: Flavor and oxidative stability of soybean, sunflower and low erucic acid rapeseed oils, JAOCS 66 (1989) 558-564.
- AOCS Cd 12b-92 (AOCS – American Oil Chemists’ Society): Sampling and analysis of commercial fats and oils: Oil Stability Index
- ISO 6886: Animal and vegetable fats and oils – Determination of oxidative stability (accelerated oxidation test)
- 2.5.1.2-1996 (JOCS – Japan Oil Chemists’ Society): Fat stability – CDM Conductometric Determination Method
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.