Targeted imaging of organs can be facilitated to distinguish healthy tissues from unhealthy tissues when contrast media are used in non-invasive diagnostics, such as X-ray and magnetic resonance imaging (MRI).
X-ray contrast media like barium and iodine compounds absorb the X-rays in order to modify the radiodensity of an organ, whereas MRI relies on the application of magnetic fields induced by charged elementary particles. The more-effective X-ray absorption by the X-ray positive contrast media than the body tissue enables them to deliver a stronger contrast.
Organically bound iodides are currently used in the preparations and are a derivative from tri-iodobenzoic acid (Figure 1). They can be safely administered both orally and intravenously, thanks to their inert nature.
They are released un-metabolized and left untreated in the wastewater treatment plants due to their poor biodegradability. Therefore, they eventually reach the environment, where they are accumulated to higher concentrations. Most European receiving waters have been shown to have X-ray contrast media at μg/L levels.
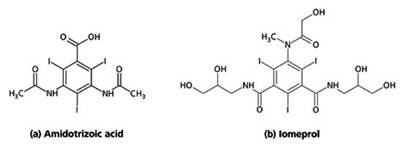
Figure 1. Chemical structures of two X-ray contrast media, (a) Amidotrizoic acid and (b) Iomeprol
Ozonization
Ozonization not only eliminates bacteria, fungi and viruses, but also provides an eco-friendly solution for degradation of persistent organic pollutants. However, nontoxic substances can be converted into lethal compounds due to ozonization, which is a major downside of this method. More research is required to gain insights into the effects of degradation of iodized X-ray contrast media and associated release of oxidation products.
Analysis
By elucidating the reaction behavior, IC-ICP/MS enables determine inorganic oxidation products like iodide and iodate and other iodized degradation products. The analysis of residual X-ray contrast media and the associated degradation products is performed by subjecting model components iomeprol and amidotrizoic acid in watery solution to various ozone doses (Figure 1). Using the results, ozonization can be optimized, and any potential risks can be predicted.
Experiments
Different oxidation levels, i.e., speciation, can be differentiated with the help of IC-ICP/MS. This means that free and bonded iodine ions can be discerned, eventually providing answer to how the iodine-containing X-ray contrast media (ICCM) gets affected by the ozonization.
Watery solutions of 21.3mg/L amidotrizoic acid (13.21mg/L iodine) and 20.4mg/L iomeprol (9.99mg/L iodine) were subjected to ozonization at 3mg/L of ozone in an ozonization reactor, followed by taking samples at time intervals of 30 minutes over a period of 3.5-4 hours.
Metrosep A Supp 3 column was used to separate the ionic, iodine-containing, degraded products under isocratic elution. An RP-18 HPLC column was used to determine the iomeprol and amidotrizoic acid under gradient elution. The standard addition method was used to quantify the degraded products as well as the X-ray contrast media.
A VG PQ ExCell ICP/MS from ThermoScientific and an 850 Professional IC from Metrohm were featured in the IC-ICP/ MS analysis system employed for quantifying the ozonization byproducts and OBPs. Table 1 lists the determination parameters of the ICP/MS analysis, and Figures 4 and 5 show the results.
Table 1. ICP/MS determination parameters
|
Mode
|
Nogas
|
|
Power
|
1200W
|
|
Atomizer
|
Concentric flow rate: 1ml/min
|
|
Flows
|
Plasma gas
|
Ar, 13l/min
|
|
|
Auxiliary gas
|
Ar, 1 l/min
|
|
|
Atomizer gas
|
Ar, 1 l/min
|
|
Detection
|
127I
|
Results
Using IC-ICP/MS, it is not possible to analyze the amidotrizoic acid itself as an evaluable peak, which is in contradiction of its ionic character. The only ozonization product that could be identified is iodate. The iodate quantification illustrated in Figure 2 revealed that the conversion of iodine into iodate was 95% after 210 minutes.
In parallel the LC-UV analysis performed on the residual amidotrizoic acid revealed that 8% of the total iodine content remained unchanged as amidotrizoic acid after 210 minutes. Recovering iodine at a rate of 103% eliminates any further chance of the formation of ozonization products.
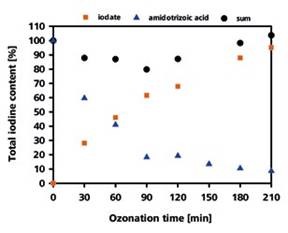
Figure 2. Recovery rate of the iodine in the form of iodate and amidotrizoic acid depending on the duration of the ozonization process
Conversely, iomeprol was slowly degraded in terms of the amount of formation of iodate, which represented only 14% of the total iodine content after 210 minutes. However, from the LC-UV analysis, the amount of iomeprol remained after 210 minutes was found to be 16%, showing only 30% total recovery rate for the iodine (Figure 3).
The remaining percentage of the iodine must be present in the form of different iodine-containing degradation products. IC-ICP/MS can be used to detect at least one of the degradation products.
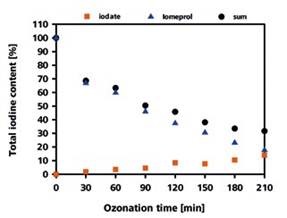
Figure 3. Recovery rate of the iodine as iodate and iomeprol depending on the duration of the ozonization process
When the chromatograms of a 100μg/l iodate/iodide standard were compared with the 60 and 210 minutes long ozonized iomeprol solution, significant deviations in the retention times can be observed. From these variations, it can be concluded that the second peak does not represent the earlier eluting iodide.
However, iomeprol’s one or more ionic degradation products could be the cause of this peak as suggested by the HPLC-ICP/MS measurement (Figure 5). This peak was accurately quantified. There was an increase in the peak area with an increase in the ozonization time until 60 minutes. After this period, the area was reduced, showing the rapid formation of the iomeprol oxidation product initially but degradation occurred upon further ozonization.
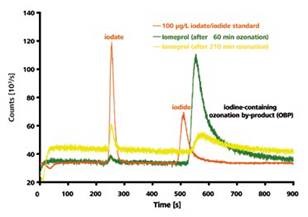
Figure 4. IC-ICP/MS-chromatogram of a 100μg/l iodate and iodide standard in comparison with an iomeprol solution after 60 and 210 minutes ozonization process, respectively. Column: Metrosep A Supp 3 - 250/4.0; eluent: 6.8mmol/l NaHCO3 and 7.2mmol/I Na2CO3, 1ml/min; Column temperature: 30°C; Sample volume: 20μl.
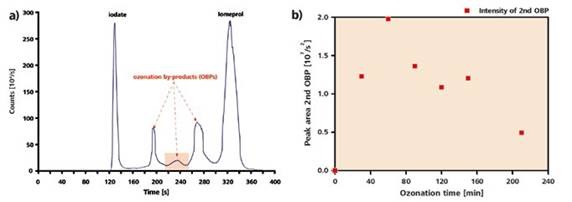
Figure 5. a) HPLC-ICP/MS chromatogram of an iomeprol solution after a 120 minutes ozonization. Column: Phenomenex Envirosep-PP 125 × 4.6mm, Eluent A: 0.1% formic acid in water (v/v), Eluent B: acetonitrile, 0.5ml/min, gradient: 0-0.5min: 95% A, 5% B, 0.5-5.7min: 60% A, 40% B; 5.7-10min: 95% A, 5% B. b) course of the peak area of the second OBP-peak (1:100 dilution) depending on the ozonization time.
Conclusion
The IC-ICP/MS analysis results have shown the possibility of determining the efficiency of the ozonization process of iodized X-ray contrast media by measuring the quantity of iodate formed. Most of the amidotrizoic acid was degraded into iodate upon ozonization for 210 minutes. However, the amount of iomeprol remained was found to be 16% when subjected to the same ozonization conditions.
The amount of iodate in the absence of iodide was only 14%. Unidentified peaks observed in the ion chromatogram suggested the presence of other iodine-containing degradation products. Therefore, the intact iodized X-ray contrast media cannot be documented under the selected ion chromatographic conditions.
Acknowledgements
Produced from materials authored by Peter Pfundstein1, Christian Martin1, Wolfgang Schulz2, Wolfram Seitz2, Katinka Meike Ruth3, Andrea Wille3, Alfred Steinbach3 and Dirk Flottmann1 from:
1Hochschule Aalen
2Zweckverband Landeswasserversorgung, Betriebsund Forschungslaboratorium, Langenau,
3Metrohm International Headquarters, Ionenstrasse, Herisau, Schweiz
References
Seitz W. et al.: Chemie in Labor und Biotechnik, Heft 12, 456–460 (2004)
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.