Residual moisture in lyophilized pharmaceutical peptides is quantified for effective quality control in the pharmaceutical industry. Routine analyses are performed for process control and to ensure that production lots meet required specifications. Such measurements are necessary for development, such as during stability studies and optimizing the freeze-drying process (lyophilization).
Although widely used for moisture determination in routine analysis, Karl Fischer titration is time-consuming and has an analysis process that destroys the sample.
Near-infrared spectroscopy (NIRS) is a fast, reagentless, and non-destructive method for determining the moisture content in lyophilized pharmaceutical products.
Experimental conditions
A Metrohm NIRS XDS OptiProbe Analyzer was used in combination with the 815 Robotic Sample Processor for sample collection. The attached large sample rack enabled the automatic measurement of up to 62 samples in a series. In this instance, 17 spectra of samples with varying moisture were collected, and KF-titration obtained reference values.
The spectra and lab values data set was split into a calibration set of 11 samples and a validation set of six samples. A maximum distance in wavelength space algorithm was used to perform an outlier section on pre-treated spectra (2nd derivative).
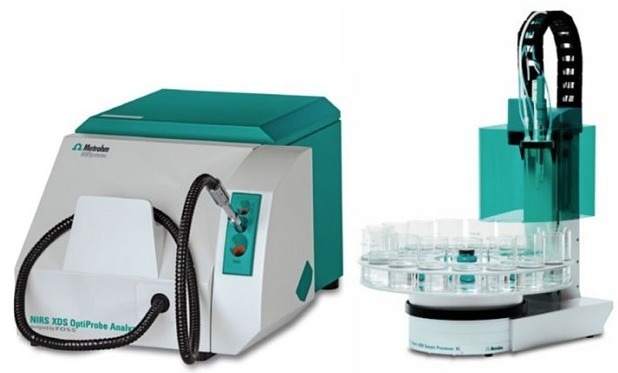
Figure 1. The NIRS XDS OptiProbe Analyzer and the 815 Robotic Sample Processor. Image Credit: Metrohm USA Inc.
Source: Metrohm USA Inc.
| Pre-processing |
Algorithm |
Validation type |
| 2nd derivative |
PLS |
Independent validation |
Figure 2. Protein samples measured with varying water content. Image Credit: Metrohm USA Inc.
Result and conclusion
The resulting correlation graph reveals a very high correlation (R2 = 0.99) between moisture predicted by the KF-titration primary method and NIRS. SEC and SEV values are in the range of 0.060%, showing that NIRS is a sensitive technique suitable for moisture determination.
Source: Metrohm USA Inc.
| # Factors |
R2 |
SEC |
SEV |
| 2 |
0.99 |
0.054% |
0.061% |
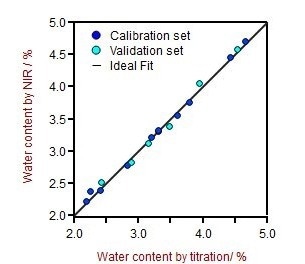
Figure 3. Correlation graph for moisture predicted by NIRS vs titration. Image Credit: Metrohm USA Inc.
About Metrohm USA Inc.
Metrohm is a worldwide leading manufacturer of precision instruments for chemical analysis. In the field of electrochemical ion analysis we have been the unchallenged world number one for many years. But they offer much more than just instruments.
In their laboratories they develop tailor-made applications that help customers to safeguard the quality of their products, to comply with regulations, and to optimize processes.
They provide various temperature sensors and optical sensors for titration with photometric endpoint recognition.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.