Common pharmacopoeias acknowledge near-infrared spectroscopy (NIRS) as a secondary method for non-destructive, reliable and rapid analysis in pharmaceutical manufacturing. The pharmaceutical industry is unique in terms of the demands made on it to implement extensive regulations. Important decisions regarding quality assurance are largely made based on regulatory requirements and guidelines.
Quality control plays a major role in the pharmaceutical sector. Aside from being robust and reliable, the quality control methods must be efficient as possible. Therefore, time-saving secondary techniques have attracted a great deal of interest from regulatory authorities and the pharmaceutical industry.
Since early 1960,[1] the pharmaceutical industry has been exploring and using the secondary analytical method NIRS for process control, the identification of raw materials and quality assurance of final products. In addition to being fast and non-destructive, NIRS needs little to no sample preparation and it obtains information on physical and chemical properties of the sample in each measurement.
Using the data obtained in a single measurement, one can determine multiple parameters, either quantitatively or qualitatively. With its non-destructive way of performing measurements and short measuring times, the full potential of NIRS unfolds, especially in process control [2].
The U.S. Food and Drug Administration (FDA), guidelines by the European Medicines Agency (EMA), the Process Analytical Technologies (PAT) initiative, and the International Conference on Harmonization in the standards (ICH) Q8(R2), ICH Q9 and ICH Q10 [1] have positioned NIRS as “a highly relevant tool for achieving control when built-in quality is preferred over quality by testing.” This article shows how Metrohm’s NIRS solutions conform to regulatory requirements.
NIRS applications
As a versatile analysis method, NIRS can be applied to a wide range of applications across the pharmaceutical manufacturing process. In the following sections, some example applications will be described, with a focus on their advantages for production and their regulatory compliance.
Incoming materials inspection
All incoming materials must be tested for conformity and for verification of the identity, according to EU GMP 8 and FDA CFR 211.84, which results in large amounts of samples. The spectrometer product portfolio from Metrohm provides a suitable solution for convenient inspection of incoming materials, whether they are analyzed directly in the warehouse, in the QC laboratory or in the weighting area.[3, 4]
Inline/online process control
Less rework time is spent and less out of specification products are generated with inline/online process control. Using Metrohm’s NIRS inline/online analyzers, real-time monitoring and optimization of, for example, viable cell density or drying processes is possible. During the production process, residual solvent and water content in granulates and powders, such as in lyophilized products, can be reliably determined. [3, 5–7]
Atline/offline process and product development
Atline and offline processes such as intermediate and product assays or blending and granulation can be monitored using Metrohm’s NIRS Analyzers. [6, 7, 9] Blend homogeneity and, owing to real-time process monitoring, optimum granulation time can be evaluated. In addition, the uniformity of content in solid dosage forms (capsules and tablets) as well as tablet characteristics such as stability and hardness, can be established [10-14].

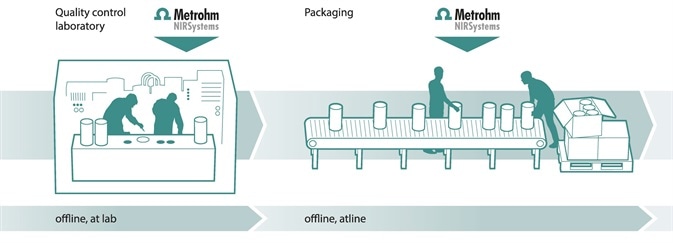
Quality assurance of finished products
Quality assurance of finished products, such as content determination in capsules, tablets, gels, creams and so on can be achieved with NIRS. The full transmission spectrometer, Metrohm’s NIRS XDS MasterLab also ensures accurate and reliable results when analyzing active pharmaceutical ingredients and excipients in tablets (even in blisters), to guarantee that they meet the requirements toward both purity and physical/chemical properties. [10-14]
Validation of NIRS
The method, instrument, and software must be validated if NIR spectroscopy is going to be used.[1] To ensure that requirements are completely validated and fulfilled, regulatory authorities such as the U.S. Pharmacopoeia (USP), EMA and FDA have published guidelines on how to develop, validate, submit and maintain NIRS analytical procedures. Although the validation process can be time- and effort-consuming, it does quickly pay off. NIRS enables real-time process monitoring and short analysis times that result in a high return on investment.
Software validation
Any software that meets the relevant regulations, such as EU Annex 11 (Computerized Systems) and/or FDA 21 CFR Part 11 (electronic signatures, electronic records) can be employed in any GLP/GMP environment.
Metrohm Vis-NIR spectroscopy software
All technical requirements specified in the FDA 21 CFR Part 11 and the EU Annex are met by the Metrohm Vis-NIR spectroscopy software Vision Air.
Figure 1. Report view in Vision Air
Instrument qualification
In accordance with the USP <1058> and GMP/GLP, the validation process of instruments involves three phases, as follows:
- Installation qualification (IQ)
- Operational qualification (OQ)
- Performance qualification (PQ)
IQ: According to USP<1119>: The IQ requirements help ensure that the hardware and software are installed according to vendor and safety specifications at the desired location. [15]
OQ: According to USP<1119>: The instrument’s performance is controlled with respect to external certified standards to verify that the system operates within target specifications.
The purpose of OQ is to ensure that an instrument is suitable for its intended application. (…) Similar to any spectrophotometric device, NIR instruments need to be qualified for both wavelength and photometric scale. Maximum and reduced light-flux noise tests are also included [15].
PQ: According to USP<1119>: “A quality to fit to an initial scan or group of scans included in the operational qualification is employed. In such an analysis, it is assumed that reference standard spectra collected on a new or a newly repaired, properly operating instrument represent the best ones available. Comparisons of spectra taken over time on the identical reference standards from the basis for evaluating the long-term stability of an NIR measurement system. The objective is to ensure that no wavelength calibration shift of change in sensitivity occurs during ongoing analysis.” [15]. Generally, laboratory personnel carry out PQ [1].
Metrohm qualification
All requirements demanded by various governing bodies (USP<1058>, USP<1119>, GAMP, 21 CFR Part 11, PIC/S, etc.) are met by Metrohm’s Analytical Instrument Qualification for NIRS instruments. Metrohm is renowned for its high-quality support which includes professional installation and the startup of new instruments in compliance with Installation Qualification (IQ) and assures that Metrohm NIRS instruments fulfill Operational Qualification (OQ) requirements, including full documentation. Instrument performance certification is provided by Metrohm, as well as work-related user training and certification.

Method validation
Once the hardware and software are validated, the methods are developed and validated. Method validation is mainly done to prove and guarantee the suitability of an analytical procedure.[2] Transparent documentation is required for every step of the method development, validation and transfer. According to Ciurczak, the development laboratory must give the following documentation to the designated laboratory or end user:
- A written procedure
- A method validation report
- System suitability criteria [1]
Summary and support
NIRS is an established secondary analysis technique developed for offline, atline, online, and inline applications in the pharmaceutical industry. Once validated, NIRS is a cost- and time-effective technique, offering a high return on investment and paying for itself quickly.
Pharmaceutical analyses that comply with common regulations are easily enabled by Metrohm’s NIRS solutions and software. Users who implement a Metrohm NIRS solution benefit from extensive support in the development of methods and applications, amongst others.
Metrohm’s method validation
Vision Air Complete — Metrohm's Vis-NIR spectroscopy software — has method validation features. The chemometric software Vision along with supported third-party tools such as PLS_Toolbox by Eigenvector Research and Unscrambler by CAMO allow users to develop and validate identification, qualification and quantification methods.


Figure 2. Report of the USP Wavelength Accuracy Test from Vision software (top) and used Metrohm NIRS XDS SmartProbe Analyzer with standard (bottom).
Metrohm NIRS applications
A broad range of application support is offered by Metrohm for the pharmaceutical industry, worldwide.
References
- E.W. Ciurczak and B. Igne, Pharmaceutical and Medical Applications of Near-Infrared Spectroscopy (CRC Press, Boca Raton, Florida, 2015).
- N. Broad, P. Graham, R. Hailey, A. Hardy, S. Holland, S. Hughes, D. Lee, K. Prebble, N. Salton and P. Warren, Guidelines for the Development and Validation of Near-Infrared Spectroscopic Methods in the Pharmaceutical Industry, Handbook of Vibrational Spectroscopy (John Wiley & Sons, Chichester, 2002)
- Using NIR as an identification technique of raw materials and product materials, Application Bulletin AB-410
- Identification of pharmaceutical raw materials, Application Bulletin AB-410
- Robert Mattes et al., Monitoring viable cell density in bioreactors using near-infrared spectroscpy, BioProcessing Journal, 2010
- Near-infrared spectroscopy for monitoring a single-pot granulator, Application Note AN-NIR-016
- Monitoring the purity of recovered solvents with NIRS, Application Note AN-NIR-021
- Analysis of residual moisture in a lyophilized pharmaceutical product by near-infrared spectroscopy (NIRS), Application Bulletin AB-358
- Following the progress of pharmaceutical mixing studies using near-infrared spectroscopy, Application Note AN-NIR-014
- Nondestructive, single tablet analysis using the NIRS XDS RapidContent Analyzer, Application Note AN-NIR-002
- Nondestructive, single tablet analysis using the NIRS RapidContent Analyzer, Application Note AN-NIR-002
- NIRS "predictive model" for the release of pharmaceutical active ingredients from solid dosage forms, Application Note AN-NIR-017
- Near-infrared (NIR) assay and content uniformity of tablets, Application Note AN-NIR-018
- Determination of active ingredients in solid (pharmaceutical) dosage forms utilizing solid-state standard additions, Application Note AN-NIR-001
- Near-Infrared Spectroscopy, <1119>, USP 39 (2016)
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.