Arsenic (As) is a metallic element that occurs naturally in soil and ores in the environment. It can exist in both inorganic and organic forms, but inorganic arsenic, whether introduced anthropogenically or naturally occurring, is typically in the form of arsenite As(III), which is partly reduced, or arsenate As(V), which is completely oxidized.
Chronic exposure to inorganic arsenic is associated with excess lung, skin, liver, kidney, and bladder cancers in humans. Both arsenite and arsenate are genotoxic and capable of inducing sister chromatid exchange and chromosome aberrations in human and rodent cells. Arsenite, in this context, is more potent than arsenate, by approximately one order of magnitude.
The two forms of inorganic arsenic affect the function of pulmonary alveolar macrophage at noncytotoxic concentrations, with arsenite being more potent than arsenate. Following intratracheal instillation in hamster lungs, both forms produce tumors (Saranko, C. J.; 1998).
Inorganic arsenic species in dietary supplements using gradient ion chromatography and IC-ICP/MS
Dietary supplements are generally regarded as beneficial to health and free of toxic side effects, but studies have demonstrated that high levels of toxic substances are present in certain supplements. Arsenic is regarded as one of the elements of primary concern due to its toxicity. Arsenic contamination of dietary supplements mainly occurs as a result of the plant materials used in the ingredients.
Both anthropogenic and natural activities cause arsenic to be released into the environment. It is taken up by plants, where it builds up in edible parts of the plants. Arsenic should be assessed based on the determination of its individual species, because it is the chemical form that influences the toxicity of the element.
In this analysis, a microwave-enhanced protocol was used to simultaneously extract organic and inorganic arsenic species from dietary supplements and the species were then determined by the IC-ICP/MS method. Baseline separation of the species was achieved using high-pressure-gradient IC with a Hamilton PRP-X200 cation exchange column and these species were then injected into an Agilent 7700 ICP/MS instrument.
Using an 800 Dosino, a post-column internal standard was injected. The IC and ICP/MS and IC instruments were synchronized using remote signal. The sample loading and gradient program were controlled by MagIC Net software, and data manipulation and handling were controlled by Agilent MassHunter software.
Results
Arsenobetaine (AsB), As(III), As(V), dimethylarsinic acid (DMA), monomethylarsonic acid (MMA) and trimethylarsine oxide (TMAO) were separated under chromatographic conditions. Although TMA and AsC were isolated from the other species, they were not resolved from one another, as depicted in Figure 1. The optimized protocol was used to analyze 10 widely used prenatal and children’s dietary supplements.
It was observed that the supplements had total arsenic contents in the range of 59–531 ng/g concentration. IC-ICP/MS analysis showed that that DMA and As(III) were present in all the supplementary products. Two of the supplements contained As(V) and one product contained an unknown species of arsenic, as shown in Figure 2.
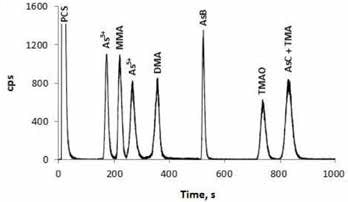
Figure 1. Standard solution containing 10.0 ng/g arsenic per species, conditions as below (Figure 2).
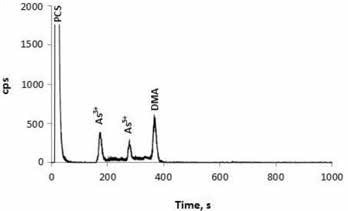
Figure 2. Microwave extract generated from a prenatal supplement prepared from plant materials. The supplement was available in hard-pressed powder (tablet) form. Column: Hamilton PRP-X200 cation-exchange column; eluent A 1.0 mmol/L HNO3 (pH 2.5); eluent B: 2.0 mmol/L HNO3, 20.0 mmol/L NH4NO3 (pH 2.5); flow rate: 0.9 mL/min; m/z 75 9.
Mercury and arsenic speciation analysis by IC-ICP/MS
This article describes the application of the IC-ICP/MS method for determining inorganic and organic mercury and arsenic compounds. Traditional speciation analysis is used to determine arsenic species (monoisotopic), as these are not prone to interconversion.
Results
The IC-ICP/MS method can be used to separate and accurately identify different arsenic species in both organic and inorganic organic forms, as illustrated in Figure 3.
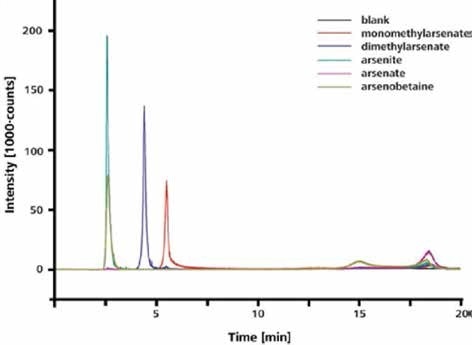
Figure 3. Separation and detection of arsenite, dimethylarsenate, monomethylarsenate, and arsenate. Column: Metrosep A Supp 15 - 150/4.0; eluent: 8 mmol/L NH4NO3 (pH 8); flow rate 0.7 mL/min; m/z 75
Simultaneous speciation of arsenic and selenium species in petroleum refinery aqueous streams
The study investigated the quantitative speciation of As and selenium (Se) in the streamwaters of a refining process.
Results
Three inorganic Se species - selenocyanate (SeCN-), selenate Se(VI) and selenite Se(IV) - and four arsenic species - As(V), As(III), DMA and MMA - were isolated in a single run by IC. This was done by using gradient elution with 100 mmol/L NH4NO3, pH 8.5, adjusted by addition of NH3 as eluent, as shown in Figures 4 and 5.
It was observed that repeatabilities of peak position and peak area evaluation were better than 1% and about 3%, respectively. Defined as 3× baseline noise, detection limits were 56, 75, and 81 ng/L for Se(VI), SeCN-, Se(IV), respectively, and 16, 19, 22, and 25 ng/L for DMA, As(V), As(III), and MMA, respectively.
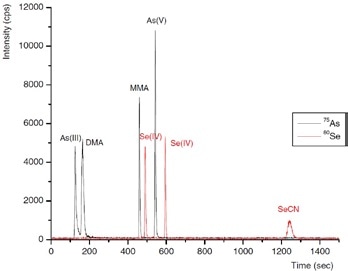
Figure 4. Separation of the arsenic species As(III), DMA, MMA, and As(V), and the selenium species Se(IV), Se(VI), and SeCN− (5 ng of each species). Conditions as Figure 5.
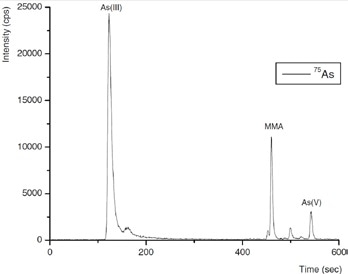
Figure 5. Arsenic species in a typical process wastewater inlet sample. Column: Metrosep A Supp 10 - 250/4.0; eluent: 100 mmol/L NH4NO3 (pH 8.5, adjusted by addition of NH3); flow rate: 1 mL/min using gradient elution; m/z 75–83.
Simultaneous determination of arsenic and selenium species in fish tissues using IC-ICP/MS
For simultaneous extraction of Se and arsenic As species from fish tissues, a microwave-assisted enzymatic extraction (MAEE) method was developed.
Results
Two reference materials, BCR- 627 and DOLT-3, were analyzed to validate the accuracy of the extraction protocol developed. In these reference materials, the extraction recoveries ranged between 82% and 94% for As. The major species detected in fish tissues were selenomethionine (SeMet) and AsB. In the fish extracts that were examined, the total number of arsenic species detected was in good agreement with the total As extracted, as shown in Figure 6.
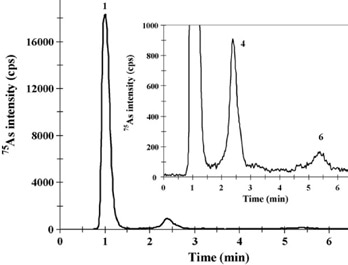
Figure 6. IC-ICP/MS profile of enzymatic extracts of (left) BCR-627 and (right) DOLT-3 obtained by MAEE. Peak identification of the right-hand chromatogram: (1) AsBet, (4) DMA, and (6) As(V). Column: Metrosep Anion Dual 3 - 100/4.0, Metrosep Anion Dual 3 guard; eluent A: 5 mmol/L NH4NO3; eluent B: 50 mmol/L NH4NO3, 2% (v/v) methanol (pH 8.7); flow rate: 1 mL/min; m/z 75, 77, 82.
Arsenic – further applications with IC-ICP/MS
- Study on analytical methods for inorganic arsenic speciation in soil by IC-ICP/MS. Mun, S. H.; Lee, B. J.; Kim, H. S.; Cho, M. K.; Sung, J. Y. (2015)
- Sulfur redox chemistry governs diurnal antimony and arsenic cycles at Champagne Pool, Waiotapu, New Zealand. Ullrich, M. K.; Pope, J. G.; Seward, T. M.; Wilson, N.; Planer-Friedrich, B. (2013) J. Volcanol. Geotherm. Res. 262, 164–177 Determination of arsenic and selenium species in drinking water applying IC-ICP/MS Application Note AN-M-010
References
- Aschner, M.; Syversen, T. (2005) Methylmercury: Recent advances in the understanding of its neurotoxicity. Ther. Drug Monit. 27, 278–283.
- Bianchi, A.; Calabi, L.; Corana, F.; Fontana, S.; Losi, Maiocchi, A.; Paleari, L.; Valtancoli, B. (2000) Thermodynamic and structural properties of Gd(III) complexes with polyaminopolycarboxylic ligands: Basic compounds for the development of MRI contrast agents. Coord. Chem. Rev. 204, 309–393
- Burger, J.; Gochfeld, M. (2005) Heavy metals in commercial fish in New Jersey. Environ. Res. 99(3), 403–412
- Dietary Supplement Fact Sheet: Selenium. US National Institutes of Health; Office of Dietary Supplements. Retrieved Oct 11, 2016.
- Gochfeld, M.; Burger, J. (2005) Good fish/bad fish: A composite benefit–risk by dose curve. Neurotoxicology 26(4), 511–520
- Herrmann, T. Einsatz der On-line-Kopplung von Ionenchromatographie und ICP-MS zur Bestimmung von Anionen. Diploma thesis, Philipps-Universität Marburg, Germany, 2006
- Knöll, J. Ph.D. Ultratrace determination of aminopolycarboxylic acid based chelating agents using inverse on-line coupling of IC with ICP-MS. PhD thesis, Philipps-Universität Marburg, Germany, 2013
- Knöll, J.; Seubert, A. (2012) Indirect ultra trace determination of aminopolycarboxylic acids in surface water using ion exchange chromatography coupled on-line to inductively coupled plasma mass spectrometry. Journal of Chromatography A 1270, 219-224.
- Kümmerer, K.; Ed. Pharmaceuticals in the environment: Sources, fate, effects, and risks. Springer-Verlag Berlin Heidelberg, 2008; 3rd ed.
- Künnemeyer, J.; Terborg, L.; Meermann, B.; Brauckmann, C.; Möller, I.; Scheffer, A.; Karst, U. (2009) Speciation analysis of gadolinium chelates in hospital effluents and wastewater treatment plant sewage by a novel HILIC/ICP-MS method. Environ. Sci. Technol. 43(8), 2884–2890.
- Künnemeyer, J.; Terborg, L.; Nowak, S.; Telgmann, L.; Tokmak, F.; Krämer, B. K.; Günsel, A.; Wiesmüller, G. A.; Waldeck, J.; Bremer, C.; Karst, U. (2009) Analysis of the contrast agent Magnevist and its transmetalation products in blood plasma by capillary electrophoresis / electrospray ionization time-of-flight mass spectrometry. Anal. Chem. 81(9), 3600–3607
- Levenson, C. W.; Axelrad, D. M. (2006) Too much of a good thing? Update on fish consumption and mercury exposure Nutr. Rev. 64(3), 139–145.
- Montaser, A.; Golightly, D. W.; Eds. Inductively coupled plasmas in analytical atomic spectrometry. VCH Publishers, Inc.: New York, 1992.
- Rahman, G. M. M.; Martone, N.; 'Skip' Kingston, H. M. (2012) Determination of hexavalent chromium in NIST SRM 2701 by speciated isotope dilution mass spectrometry (EPA Method 6800) using IC-ICP-MS. In Handbook of hyphenated ICP-MS applications, 2nd edition; Agilent, 2012; pp 33–35. http://www.agilent.com/cs/library/applications/5990-9473EN_icpmsSpeciationHB_lr.pdf (accessed Oct 7, 2016)
- Saranko, C. J. et al. Fact Report for toxicity of arsenite and arsenate, Florida Dept. of Health, Nov 6, 1998
- Schedlbauer, O. F.; Heumann, K. G. (2000) Biomethylation of thallium by bacteria and first determination of biogenic dimethylthallium in the ocean. Appl. Organomet. Chem.14(6), 330-340
- Wilber, C. G. (1980). Toxicology of selenium: A review.Clin. Toxicol. 17 (2), 171–230.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.