Cost-effective, high-accuracy drug analysis is essential for regular QC purposes and for determination of counterfeit medicines that are potentially harmful. Near-infrared spectroscopy (NIRS) is a valuable tool for pharmaceutical analysis, as it requires minimal labor resources and analysis time, and has the ability to perform completely automated online analyses and to analyze various substances simultaneously. This article discusses some of the applications of NIRS.
Quality by Design (QbD) and Process Analytical Technology (PAT)
The US Food and Drug Administration (FDA) has stepped up focus on QbD and PAT concepts, which are designed for improving the efficiency of drug development and production. A design replaces the trial-and-error procedure in QbD, and is continuously fine-tuned from the beginning to make a drug suitable for the patient population and for various ways in which it can be administered, thus enabling a production process capable of delivering the desired results from the beginning.
PAT is essential for a successful QbD approach, providing real-time monitoring of production. As a result, the desired quality can be achieved by allowing modifications during the process itself. Moreover, the process and product can be understood in a better manner.
NIRS Method
A number of publications are available that describe the role of NIRS in controlling the quality prior to, during, and after production. Extensive physical and chemical data can be extracted from the peaks and troughs of the NIR spectra using chemometric methods. NIRS has the ability to determine five active pharmaceutical ingredients (APIs) present in tablets intended for the relief of flu symptoms, namely, chlorphenamine maleate, caffeine, dextromethorphan hydrobromide, ascorbic acid, and paracetamol.
The Pharmaceutical Analytical Sciences Group (PASG), the European Medicines Agency (EMA), and the International Conference on Harmonization (ICH) provide guidelines for the validation of the NIRS method, making it an accepted alternative to the laborious reference method, which involves separate analyses with HPLC and titration.
Extracting Data from NIR Spectra
These tablets have an NIR spectrum nearly identical to that of sucrose, which is a pure pharmaceutical excipient with a concentration much higher than any of the APIs present in the tablets. Slight changes in the spectrum are also caused by the active ingredients, providing a basis for determination and quantification of the substances with the help of appropriate models. However, suitable samples are required to develop meaningful models. Performing principal component analysis (PCA) for all the spectra is the first step for identifying the suitable samples.
The samples identified by PCA have optimum spectral variability and cover the whole range of concentration of all APIs. Calibration models can be developed using these samples to establish a correlation between the NIR spectra and the concentrations measured with reference methods.
Partial least squares (PLS) regression is a chemometric method commonly used for the development of a model for each analyte. By applying the models to the sample spectrum, the concentration of each active ingredient can be predicted. Considerable amount of time and costs can be saved using this proven method, thanks to shortened analysis time, absence of reagents, and elimination of waste.
Determining Counterfeit Medicines
NIRS not only analyzes active ingredient content but also helps determine counterfeit medicines (Figure 1). First-world countries may not face many problems with counterfeit medicines, but risks are much higher for the patients and agents in the developing countries. The classification of tablets consisting of orphenadrine, caffeine, and metamizole in a non-destructive and rapid manner using NIRS was described in a 2013 publication. Large sample volume can be used in this method.

Figure 1. Besides routine analyses of active ingredient content, NIRS can also be used as a fast and cost-effective way of testing whether drugs are genuine.
The method developed by the authors of this study was based on four preparations obtained from various manufacturers. One of the preparations was defined as the reference, and models were then developed for distinguishing the reference product from the remaining three products using successive projection algorithm-linear discriminant analysis (SPA-LDA), genetic algorithm- linear discriminant analysis (GA-LDA), and soft independent modeling of class analogies (SIMCA).
The models generated from these algorithms had different levels of complexity. The spectra’s entire measured wavelength range was used for modeling with the SIMCA algorithm. However, 2 and 12 selected wavelengths were only used for modeling with SPA-LDA and GA-LDA, respectively. It was shown that all three models were able to classify the preparations with 100% accuracy. The modeling offered by the SPA-LDA and GA-LDA algorithms is rapid and cost-effective, and can provide reliable predictions through appropriate validation. The results of SPA-LDA modeling are shown in Figure 2.
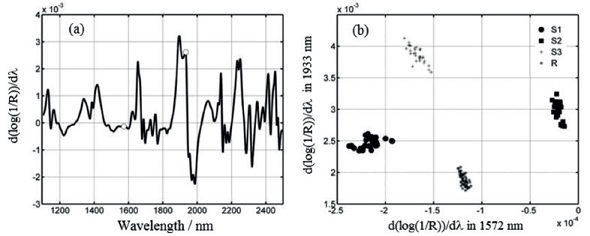
Figure 2. Results of SPA-LDA modeling. a) Derivative NIR spectrum; the wavelengths used for modeling (1,572 and 1,933nm) are marked with circles; b) bivariate plot of 150 samples classified on the basis of the model.
Conclusion
The FDA’s PAT initiative largely contributes to the increasing adoption of NIRS in the pharmaceutical industry. NIRS is a proven method for process and quality control, and is expected to contribute further in improving the efficiency of drug development and production. Identifying pharmaceutical products and their ingredients, such as excipients and APIs, is possible using NIRS in conjunction with an appropriate model. Besides allowing quality control at the production line rapidly and directly, the NIRS method helps determine counterfeits.
References
- Blanco, M. and M. Alcalá (2006) Eu. J. Pharm. Sci. 27, 280–286
- Melo, C. A. D. et al. (2013) J. Braz. Chem. Soc. 24(6), 991–997
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.