Obtaining reliable analysis results is very critical in the pharmaceutical industry. Sample preparation and the following titration steps are completely automated by the reliable 815 Robotic Soliprep system (Figure 1) from Metrohm. Solid samples like tablets can be effectively analyzed with the help of the in-built high-frequency homogenizer present in this system. The content determination results are highly accurate and show minimal standard deviations.

Figure 1. The 815 Robotic Titration Soliprep with built-in high-frequency homogenizer processes your samples fully automatically and analyzes them by titration.
The most important aspect in the manufacture of tablet-form pharmaceuticals is that each tablet present in a batch must have a concentration of active substance same as mentioned on the packaging, within narrow limits. Active substances can be accurately determined using a number of analytical methods.
Titrimetric determination methods are the basis for many SOPs (standard operating procedures) outlined in the European Pharmacopoeia and the United States Pharmacopeia. If the titrimetric quantification of active substances is allowed by its chemical properties, it is possible to avoid the use of LC-MS or GC-MS methods, which are both laborious and expensive.
Meticulous and reproducible sample preparation methods provide highly precise results. It may be required to perform various sample preparation steps before the start of analysis based on the coating and shape of tablets, fillers contained in them, and their active substance concentration.
Homogenization in an appropriate solvent mixture is mostly the first step of sample preparation. The requirement for additional steps like diluting or pipetting arises based on each analysis technique. These elaborate and time-intensive steps are still manually performed in most laboratories. Moreover, manual sample preparation steps often result in contamination and carry-over due to the interferences and marginal variations associated with manual sample preparations, eventually leading to erroneous results.
Fully Automated Sample Preparation and Tablet Analysis
Automation of sample preparation helps eliminate all these issues and enables standardization of sample preparation because the handling procedures are the same for each sample. Automation proves to be useful in determining the active-substance concentration of individual tablets, and also for evaluating the quality of the entire batch through random sampling.
Apart from providing highly accurate and reproducible results, automated systems also produce higher sample throughput, minimize the consumption of reagent, and enhance the safety in labs. Automation also relieves laboratory personnel from tedious tasks so that they can focus on other tasks.
Instruments
A sample changer equipped with a built-in homogenizer, dosing units for addition of the solvent, a titrator with a combined pH electrode, and a peristaltic pump to aspirate off the contents of the beaker at the end of the measurement, constitute the fully automated system used for the quantification of active substance content in tablets. The tiamo™ titration software is used for data control and acquisition.
The following components constitute the automated setup:
- 815 Robotic Titration Soliprep
- Polytron 1300D – the built-in high-frequency homogenizer
- External rinsing station
- 2 x 800 Dosinos with dosing units
- 809 Titrando
- 772 pump unit
- 6.0229.100 Solvotrode
- Maximum of 59 sample beakers with a capacity from 50 to 100 mL
- Control and data acquisition software tiamo™
The high-frequency homogenizer is available in two versions: one with protruding blades and another is the standard version that comes with short blades (Figure 2). The aggregate length of the blades in the protruding blades homogenizer is 157mm. This version is used for samples that need to be powdered, whereas the standard version is designed for achieving homogeneity in suspensions and pastes. The Polytron homogenizer is an optimum fit for Metrohm’s requirements.

Figure 2. Aggregate of the high-frequency homogenizer with short blades (left) and with protruding blades (right)
Sample Preparation and Titration
A very small time is enough to complete the fully automated sample preparation and subsequent titrimetric content determination of tablet ingredients. Steps such as sample weighing, filling in the sample table, sample positioning in the sample changer rack are manually performed. The rest of the steps is automated by the system, without the need for operator intervention.
Analysis of substances containing a phenolic hydroxyl group by titrating with a strong base is illustrated in this article.
First, the required number of tablets is weighed out into the sample beaker, and then the required beakers are placed on the rack, and the relevant details, such as weight and name of the sample, and position of the rack, are entered into the sample table of the tiamo™ titration software.
At the first workstation, sample preparation is started by automatically treating the sample with 60mL methanol by a Dosino with the dosing unit that is placed directly on the reagent bottle to save space. The next step is powdering one or three tablets by the in-built Polytron high-frequency homogenizer (Figure 3) at a speed of 25,000min-1 in 90 to 120 seconds.

Figure 3. The Polytron high-frequency homogenizer permits the fully automated processing of solid samples such as tablets. After solvent addition the tablets are homogenized directly in the sample beaker
The hardness and the solubility of the tablets being analyzed determine the time for pulverization. After homogenization of the sample 10mL of ultrapure water is added to it, which is the last step in sample processing. At the second workstation using the 809 Titrando and Solvotrode, the sample is then titrated with NaOH solution (c(NaOH) = 1 mol/L) immediately.
Simultaneously, the homogenizer is automatically rinsed and cleaned thoroughly at the external rinsing station. After titration is completed, the beaker is emptied and the pH electrode is rinsed, then the contents of the beaker are aspirated by the 772 Pump Unit.
Application Example: Active-Substance Determination
The following example describes the detection of the active-substance content of tablets used for bringing down increased uric acid levels in blood. Phenolic hydroxyl group is contained in the active substance, which is consequently a weak acid with strength nearly equal to that of acetic acid. Due to resonance stabilization, the proton released by the compound delocalizes the negative charge of the oxygen atom throughout the entire ring.
The possibility of the formation of the more stable anion due to deprotonization is higher. The active-substance content can be conveniently determined by titrating with NaOH solution (Figure 4). The entire process is completed within 8 minutes, which includes the sample preparation time. The results of the ten titrations on a single tablet are listed in Table 1.
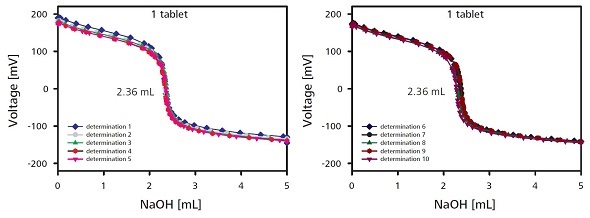
Figure 4. Titration curves for the determination of the content with one sample tablet. For reasons of clarity the titration curves of the 10 individual measurements are shown in two plots.
Table 1. Results of the ten titrations, each with one sample tablet
|
Determination
No.
|
Sample
weight mg
|
Titrant consumption
mL NaOH
|
Active substance
|
|
%
|
mg/tabl.
|
|
1
|
256.6
|
2.3523
|
38.53
|
98.9
|
|
2
|
256.6
|
2.3653
|
38.74
|
99.4
|
|
3
|
259.1
|
2.363
|
38.65
|
100.2
|
|
4
|
255.1
|
2.3436
|
38.61
|
98.5
|
|
5
|
256
|
2.3763
|
39.01
|
99.9
|
|
6
|
258.4
|
2.3633
|
38.44
|
99.3
|
|
7
|
260.2
|
2.3971
|
38.72
|
100.7
|
|
8
|
260.6
|
2.3252
|
37.5
|
97.7
|
|
9
|
261
|
2.3936
|
38.54
|
100.6
|
|
10
|
249.5
|
2.2934
|
38.63
|
96.4
|
|
|
|
|
Mean value 99.2
|
|
|
|
|
RSD 1.36%
|
Evaluation
Based on the below equation, the active-substance content of a tablet is determined. The parameters required for the calculation are sample weight, titrant consumption (NaOH solution), and molarity and titer of the titrant.
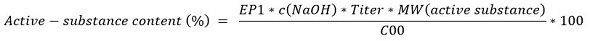
Where, EP1 is titrant consumption up to endpoint in mL; c(NaOH) is concentration of NaOH titrant in mol/L; titer of NaOH titrant (no unit); MW(active substance) molecular weight of active substance in g/mol; and C00 sample weight in mg.
Results
The active-substance content was determined using two measuring series, each consisting of 10 samples. After weighing a single tablet and three tablets for the first and second series, respectively, they were processed and subsequently titrated. An active-substance content of 99.2 and 98.7mg per tablet was determined through the 10-time titration of samples consisting of one or three tablets, respectively (Figure 5).
The use of three tablets in place of a single tablet for determination improved the relative standard deviation from 1.36 to 0.88%. The results agree well with the values provided by the manufacturer for 100mg/tablet. Table 2 provides the results of the ten titrations, each carried out with three sample tablets.
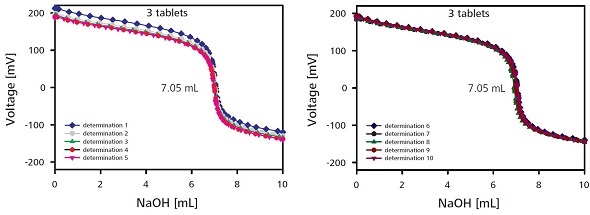
Figure 5. Titration curves for the determination of the content with three sample tablets. For reasons of clarity the titration curves of the 10 individual measurements are shown in two plots.
Table 2. Results of the ten titrations, each with three sample tablets
|
Determination
No.
|
Sample
weight mg)
|
Titrant consumption
mL NaOH
|
Active substance
|
|
%
|
mg/tabl.
|
|
1
|
769.7
|
7.0266
|
38.37
|
98.4
|
|
2
|
771.2
|
6.9992
|
38.14
|
98.1
|
|
3
|
768.2
|
7.1721
|
39.24
|
100.5
|
|
4
|
768
|
7.0305
|
38.47
|
98.5
|
|
5
|
771.4
|
7.1075
|
38.72
|
99.6
|
|
6
|
774.3
|
7.0507
|
38.27
|
98.8
|
|
7
|
772.6
|
7.015
|
38.16
|
98.3
|
|
8
|
766
|
6.9506
|
38.14
|
97.4
|
|
9
|
773.5
|
7.0211
|
38.15
|
98.4
|
|
10
|
776.6
|
7.0817
|
38.32
|
99.2
|
|
|
|
|
Mean value 98.7
|
|
|
|
|
RSD 0.88%
|
Conclusion
A maximum of 59 different samples can be automatically processed and titrated using the 815 Robotic Titration Soliprep. The in-built high-frequency homogenizer enables convenient processing of solid samples like tablets. It can be seen from the example above that the results were highly accurate and the standard deviations were comparatively low.
Furthermore, automation of the entire processing brings down the analysis time drastically. The 815 Robotic Soliprep features three interchangeable sample changers, making the system suitable for sample preparation of HPLC, IC, voltammetry or ICP in addition to titrimetric applications. Each version comes with an in-built high-frequency homogenizer.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.