High-performance liquid chromatography (HPLC) is most widely used for the analysis of dosage forms. All ingredients are routinely checked for identity and weight by an operator and a qualified pharmacist for commercial batches of solid dosage forms. A validated procedure is then used to blend the components, followed by compressing the product into tablets in accordance with standard operating procedures (SOPs) to be followed for that product.
Although the HPLC method provides promising results, laborious sample preparation required for extracting the active materials from the matrix and a time-intensive separation analysis are its major drawbacks. Since, solvents are used in both parts of the analysis, it is necessary to perform such analyses in a well-vented laboratory.
The inclusion of the preparation step often extends the average time for an assay to hours. Moreover, a fair amount of lab space is required for the HPLC equipment, which also involves additional ongoing expenses in the form of consumables, such as columns, syringes, filters, vials, solvents, and other disposables.
Separating the just-blended materials to assay for homogeneity of the actives may not be mandatory if it is possible to use an alternate, non-invasive method. One such non-intrusive, non-destructive, and rapid technique is near-infrared spectroscopy (NIRS) that extracts useful information from the combined spectra of a dosage form using chemometrics, which involve the application of computer programs for relating physical, chemical and spectral values. The absence of solvent and minimal or no sample preparation are the other advantages of the NIRS method.
Experimental Procedure
The sample analysis was performed using a NIRS XDS RapidContent analyzer, which was equipped with a centering iris for sample positioning. The reflectance mode was used for sample collection in the region of 1100–2500nm. This study used several commercial batches of procainamide HCl tablets of 500 and 750mg.
Scanning of 100 individual tablets was performed for each batch and further analysis was carried out for 10–20 individual tablets that exhibited the largest apparent spectral differences using the reference HPLC method. After appending the HPLC results to the NIR spectra, a calibration model was generated based on the regions involving the absorption of actives.
Results and Discussion
Figure 1 shows the absorbance spectra of the procainamide HCl tablets. Both surface and multiplicative scattering effects were the cause of the visible spectral differences, and most of them can be eliminated by transforming to the second derivative spectra.
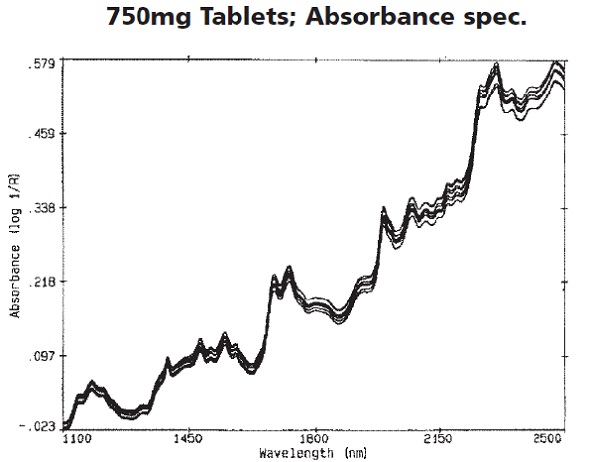
Figure 1. Absorbance spectra of the procainamide HCl tablets
Calibration was then performed using the resultant spectra shown in Figure 2. Figure 3 depicts the various regions showing the spectral differences between the active and placebo tablets.
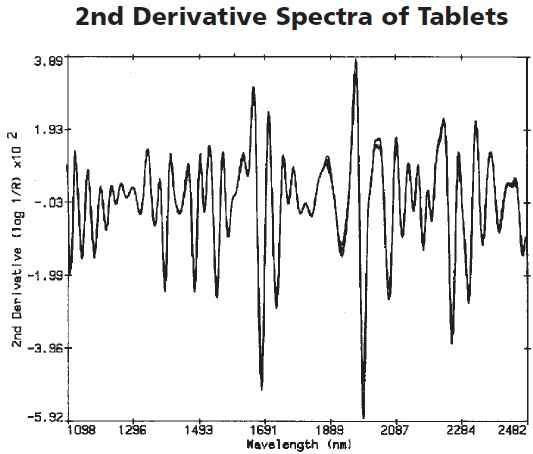
Figure 2. The second derivative spectra of the tablets
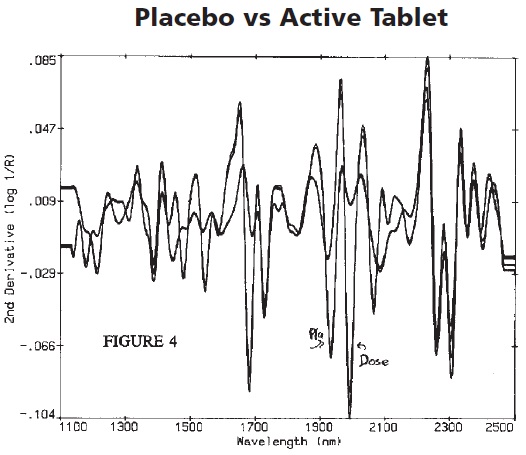
Figure 3. This image shows the numerous regions where spectral differences occur between the active and placebo tablets
The second derivative enlargement of the active, matrix, and tablet is illustrated in Figure 4. In this spectral region, most of the absorbance is contributed to the overall tablet spectrum by the active, with minimal contribution by the matrix. This spectral region is ideal for the development of calibration models. In this analysis, a batch of 750mg tablets was used to develop a calibration model at 1368nm, yielding a correlation and a standard error of calibration (SEC) of -0.84 and 1.7mg, respectively.
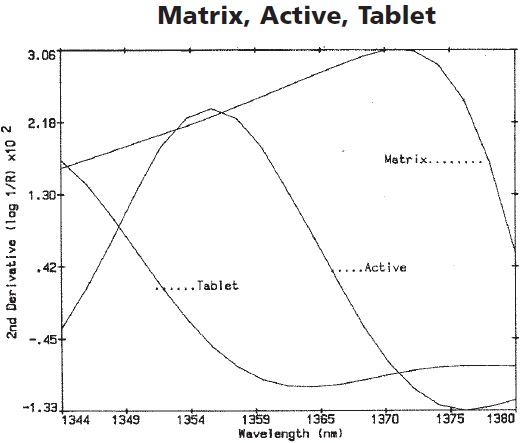
Figure 4. The second derivative enlargement of the active, matrix, and tablet
The relative residual error of 0.53% was the largest between the HPLC and NIRS. The validation was performed by selecting a second batch of 750mg tablets. The largest relative residual error and the standard error of prediction (SEP) were found to be 0.43% and 1.4mg, respectively.
The SEC value is comparable with the SEP value, thus validating the calibration model. The versatility of the NIRS method was tested further by analyzing a lot of 500mg tablets, yielding satisfactory results.
The addition of tablets with assay values greatly out of the range of the mean to the sample set generally makes the equation employed for tablet analysis more robust. Outliers rarely appear in commercial tableting process as it is typically a well-controlled process.
However, if outliers are identified, they are included to the sample set for increasing its range. Consequently, there is a continuous improvement in the equation applied for finished product release due to the addition of more diverse values to the calibration set over time.
Conclusion
For solid dosage forms, NIRS provides a rapid and non-destructive assay. Sample preparation is not required for the NIRS analysis, which is location insensitive and requires roughly 30 seconds for completion. It may be possible to perform the assay in a traditional QC environment such as laboratories, or, on-site at production lines. The latter method eliminates time-intensive processes, such as separating, labeling, shifting and assaying the product.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.