Gadolinium contrast agents, which are mainly administered via intravenous injection, exhibit excellent in vitro and in vivo stability. These agents have a half-life of about two hours. They are excreted from the body unchanged via the kidney-urine pathway and passed into wastewater.
Molecular structure of Gadobutrol (Gadovist) - gadolinium containing MRI contrast agent. Image Credits: Raimundo79/Shutterstock.com
Flocculation with Al3+ or Fe3+ salts is carried out in municipal sewage treatment plants to improve the settling behavior of suspended and dissolved colloidal materials. These trivalent metal ions that are added compete with Gd3+ ions for the organic ligands.
IC-ICP/MS analysis of gadolinium-based MRI contrast agents
Depending on the thermodynamic complex stability, addition of the trivalent metal salts may cause transmetallation or recomplexing in the MRI contrast agents, resulting in the release of toxic Gd3+ ions. Metal exchange in gadolinium chelates occurs as part of iron flocculation (Künnemeyer, J., 2009; Kümmerer, 2008).
This article examines the degree to which toxic gadolinium ions are released from MRI chelates as a result of flocculation carried out in wastewater treatment. IC-ICP/MS is used to carry out gadolinium speciation.
Results
Well-separated peaks for Magnevist and Gadovist are generated by IC-ICP/MS analysis on the anion-exchange column. Being an uncharged, polar compound, Gadovist elutes after just a few minutes, whereas the twice negatively charged Magnevist is retained more strongly and elutes much later, as shown in Figure 1.
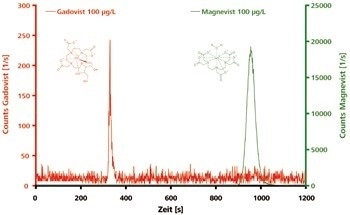
Figure 1. Chromatograms of polar and electrically neutral Gadovist and of ionic Magnevist (both 100 μg/L). Column: Metrosep A Supp 3 - 250/4.6; eluent: 7.2 mmol/L Na2CO3, 6.8 mmol/L NaHCO3; flow rate: 1.0 mL/min; m/z 156, 158, 160
The extent of transmetallation is reflected by the concentrations of the competing Gd(III) and Fe(III) ions on the polyaminopolycarboxylate ligands. This showed that displacement of gadolinium increased as the concentration of Fe3+ ions increased. This effect was stronger for Magnevist than for Gadovist. According to the higher thermodynamic stability constant of the iron(III)-DTPA complex (log K = 28.6), Magnevist (log K = 23.0) can be recomplexed to about 80% by Fe3+ concentrations of just 5 mg/L (Künnemeyer, J., 2009).
Over 90% of the chelate complexes are in the form of iron chelate complexes, with an Fe3+ concentration of 20 mg/L, whereas, Gadovist releases less than 10% of the Gd3+ ions, even at higher concentrations of Fe3+ ions. The complex center of Gadovist is effectively shielded against the competing Fe3+ ions by the crownether-like polyaminopolycarboxylic acid ligand, which is spherically arranged around the gadolinium. This largely prevents transmetallation. On the other hand, the linear DTPA ligands of Magnevist only weakly protect the central atom, so gadolinium is largely substituted by iron, resulting in the formation of the more stable iron (III) complex (Bianchi, A., 2000).
Gadolinium – further applications with IC-ICP/MS
Speciation and Isotope Dilution Analysis of Gadolinium-Based Contrast Agents in Wastewater - Telgmann, L.; Wehe, C.A.; Birka, M.; Künnemeyer, J.; Nowak, S.; Sperling, M.; Karst, U. (2012) Environ. Sci. Technol. 46(21), 11929–11936.
References
- Aschner, M.; Syversen, T. (2005) Methylmercury: Recent advances in the understanding of its neurotoxicity. Ther. Drug Monit. 27, 278–283.
- Bianchi, A.; Calabi, L.; Corana, F.; Fontana, S.; Losi, Maiocchi, A.; Paleari, L.; Valtancoli, B. (2000) Thermodynamic and structural properties of Gd(III) complexes with polyaminopolycarboxylic ligands: Basic compounds for the development of MRI contrast agents. Coord. Chem. Rev. 204, 309–393
- Burger, J.; Gochfeld, M. (2005) Heavy metals in commercial fish in New Jersey. Environ. Res. 99(3), 403–412
- Dietary Supplement Fact Sheet: Selenium. US National Institutes of Health; Office of Dietary Supplements. Retrieved Oct 11, 2016.
- Gochfeld, M.; Burger, J. (2005) Good fish/bad fish: A composite benefit–risk by dose curve. Neurotoxicology 26(4), 511–520
- Herrmann, T. Einsatz der On-line-Kopplung von Ionenchromatographie und ICP-MS zur Bestimmung von Anionen. Diploma thesis, Philipps-Universität Marburg, Germany, 2006
- Knöll, J. Ph.D. Ultratrace determination of aminopolycarboxylic acid based chelating agents using inverse on-line coupling of IC with ICP-MS. PhD thesis, Philipps-Universität Marburg, Germany, 2013
- Knöll, J.; Seubert, A. (2012) Indirect ultra trace determination of aminopolycarboxylic acids in surface water using ion exchange chromatography coupled on-line to inductively coupled plasma mass spectrometry. Journal of Chromatography A 1270, 219-224.
- Kümmerer, K.; Ed. Pharmaceuticals in the environment: Sources, fate, effects, and risks. Springer-Verlag Berlin Heidelberg, 2008; 3rd ed.
- Künnemeyer, J.; Terborg, L.; Meermann, B.; Brauckmann, C.; Möller, I.; Scheffer, A.; Karst, U. (2009) Speciation analysis of gadolinium chelates in hospital effluents and wastewater treatment plant sewage by a novel HILIC/ICP-MS method. Environ. Sci. Technol. 43(8), 2884–2890.
- Künnemeyer, J.; Terborg, L.; Nowak, S.; Telgmann, L.; Tokmak, F.; Krämer, B. K.; Günsel, A.; Wiesmüller, G. A.; Waldeck, J.; Bremer, C.; Karst, U. (2009) Analysis of the contrast agent Magnevist and its transmetalation products in blood plasma by capillary electrophoresis / electrospray ionization time-of-flight mass spectrometry. Anal. Chem. 81(9), 3600–3607
- Levenson, C. W.; Axelrad, D. M. (2006) Too much of a good thing? Update on fish consumption and mercury exposure Nutr. Rev. 64(3), 139–145.
- Montaser, A.; Golightly, D. W.; Eds. Inductively coupled plasmas in analytical atomic spectrometry. VCH Publishers, Inc.: New York, 1992.
- Rahman, G. M. M.; Martone, N.; 'Skip' Kingston, H. M. (2012) Determination of hexavalent chromium in NIST SRM 2701 by speciated isotope dilution mass spectrometry (EPA Method 6800) using IC-ICP-MS. In Handbook of hyphenated ICP-MS applications, 2nd edition; Agilent, 2012; pp 33–35. http://www.agilent.com/cs/library/applications/5990-9473EN_icpmsSpeciationHB_lr.pdf (accessed Oct 7, 2016)
- Saranko, C. J. et al. Fact Report for toxicity of arsenite and arsenate, Florida Dept. of Health, Nov 6, 1998
- Schedlbauer, O. F.; Heumann, K. G. (2000) Biomethylation of thallium by bacteria and first determination of biogenic dimethylthallium in the ocean. Appl. Organomet. Chem.14(6), 330-340
- Wilber, C. G. (1980). Toxicology of selenium: A review.Clin. Toxicol. 17 (2), 171–230.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.