This article details the application of the Metrohm Instant Raman Analyzer Mira. M-3 to identify and verify fatty acids, like those detected in cosmetics or nutraceuticals. Nutraceuticals are found in gel caps and are products extracted from food that claim to deliver additional health benefits beyond straightforward nutritional value.
As the market for personal health continues to embrace natural homeopathic treatments, a number of novel products are appearing on the market that claim benefits to the supplementation of one’s diet with vitamins and fatty acids, such as oils that provide vitamin E without raising LDL (“bad”) cholesterol. While some of these nutraceuticals are controlled by the FDA, others are not.
Despite this, it is imperative to manufacturers that their products satisfy both internal and external regulations. To develop a high quality product, it is vital to determine the identity and purity of ingredients. Expensive delays and the risk of low-quality products can be circumvented by ensuring ingredients are examined before the manufacturing process begins.
Method Description
Introduction
It is necessary to verify fatty acids employed in production during the production process. It can be tricky to distinguish exact fatty acids using Pearson’s correlation algorithm, as they can often appear similar, but verification with a p-value algorithm offers a more vigorous process through which to make sure the right material is employed in the manufacturing process.
The Metrohm Instant Raman Analyzer 3 (Mira M-3) is a handheld Raman spectrometer developed for fast, safe identification and confirmation of samples. In order to identify samples, a spectrum of the sample is measured and compared with existing spectra in a library, with the result subsequently shown with a Pearson’s correlation.
The confirmation of samples is carried out using a training set of the spectra that holds the permissible variability between diverse samples of the same material. The training set is examined with Principal Components Analysis (PCA) and reported as a percent likelihood that the sample measured is within a confidence level set by the operator.
A 95% confidence level is generally applied for material verification. While identification via a library and verification with a training set are both helpful, verification allows for the detection of minute variations in samples.
The fatty acids and fatty alcohol studied in this application note will be lauric acid (C11H23CO2H), myristic acid (C13H27CO2H), palmitic acid (C15H31CO2H), stearic acid (C17H35CO2H), and stearyl alcohol (C17H37OH).
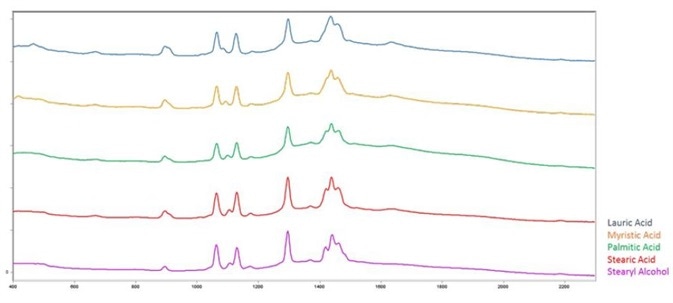
Figure 1 shows the spectra of these materials and the spectral similarities, illustrating the difficulty of differentiation on correlation alone.
Experimental
Creating an Operating Procedure (OP)
Using MiraCal software, create an OP to construct the library. Next, choose the Operating Procedures tab and generate a new OP, “Fatty Acids.” The parameters are set to laser power 5, average of 1, and auto integration time. Finally, obtain a spectrum of each sample with the OP, label each sample with care, and synchronize the data with the MiraCal software database.
Creating and Testing the Fatty Acid Library
The fatty acid library can now be set up using the samples that were saved with the “Fatty Acid” OP. Choose the Libraries tab and label the new library “Fatty Acid Library.” Add the earlier collected samples to the “Fatty Acid Library” and save it. Then, generate a new OP with the name, “Library Testing” and fix the parameters at laser power 5, average of 1, and auto integration time.
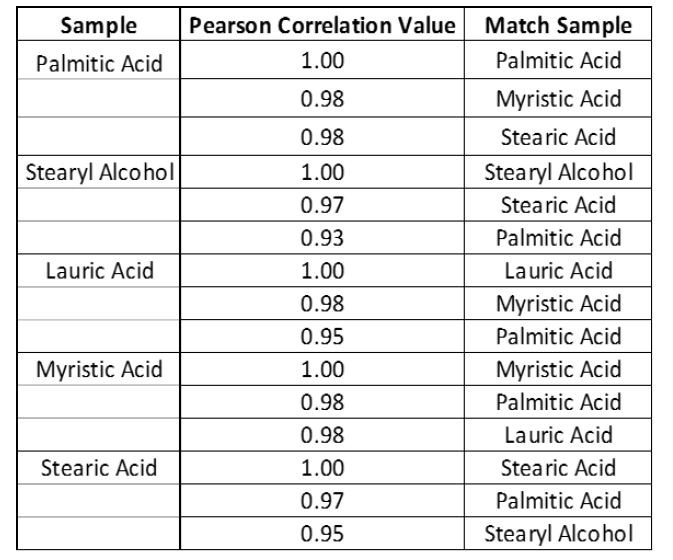
With the Evaluations tab chosen, tick the identification box and choose the “Fatty Acid Library.” Save the “Library Testing” OP and synchronize it with your system. The system can now be used to identify samples against the “Fatty Acid Library.” An illustration of match scores for each fatty acid sample is demonstrated in Table 1.
Table 1
Creating and Testing the Fatty Acid Training Set with P-Value
Choose the “Fatty Acids” OP that was set up in the earlier experiment, and go on to gather ~20 spectra of each fatty acid sample. After this is complete, link the instrument to the MiraCal software and synchronize the data to the database. Following this, you will need to produce a training set for each sample.
Choose the Training Sets tab in the software, and go on to produce new training sets for each material by inputting the sample name as the training set name and adding the ~20 spectra that were gathered in the last step. After all five training sets have been produced and saved, it is time to generate new OPs that link to each training set.
Each of the five OPs will share identical acquisition parameters: auto integration time selected, laser power 5, and average set to 1. In the Evaluation tab of the OP, tick the Verification box for each OP, and connect the corresponding training set by clicking the “Training Set” button.
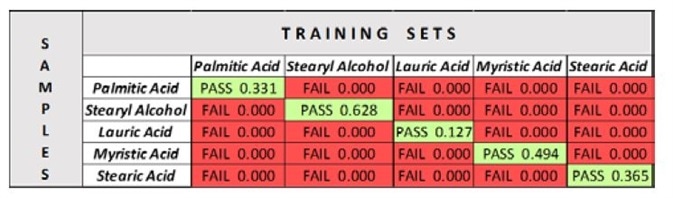
When this is complete, save each OP and synchronize the software database to add the OPs to the system. Lastly, measure a spectrum of each sample against each OP. The Pass/Fail results are logged in Table 2.
Table 2
Results and Discussion
As has been demonstrated, basic library matching (Pearson’s correlation) can sometimes fail to correctly identify a material when materials with similar properties are registered in the library. Similar materials may have match scores which vary by just 0.01-0.03 Hit Quality Index (HQI), making them tricky to interpret and resulting in lower overall confidence in the analysis (Table 1).
With verification, the sample is measured against the chosen training set, creating a positive result (“Pass”) where it falls within the set, and a negative result (“Fail”) where it falls outside of it. Through the use of verification models for each of the fatty acid samples, and the subsequent ability to test models against these samples, it can be seen that the instrument can accept or reject (“Pass” or “Fail”) distinct samples that may appear similar.
Furthermore, the results are of verification are simple to understand (Table 2). For example, palmitic acid passes with a 33.1% confidence that is within the 95% confidence interval set.
Conclusions
Where samples show significant variation in spectra, identification is useful, while for samples where spectral features appear similar, verification is a more appropriate technique. Where no existing knowledge of the sample make-up is held, correlation is employed to examine a library of known materials in an attempt to identify the sample.
Where users are already aware of what the sample is, but need to confirm the material as authentic, verification of the sample with the p-value is the ideal route. The “Pass” and “Fail” results of verification offer increased confidence in the confirmation of the sample’s identify, whereas with identification, there is a risk of high match scores with samples that share a number of similarities.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.