Chromium (Cr) species can occur in a number of oxidation states, but – Cr(III) and Cr(VI) – the trivalent and hexavalent oxidation states are the most common in the environment. The chemistry and bioavailability of the species influences its toxicity and both of these are related to its concentration and chemical form (species). Trivalent chromium is more stable and relatively non-toxic.
In the human diet, trivalent chromium is an essential trace nutrient that helps to maintain effective metabolism of lipids, glucose and proteins. Hexavalent chromium, on the other hand, is regarded as carcinogenic and toxic, as it can diffuse through the cell membrane and oxidize biological molecules by (Rahman, G. M. M. et al.; 2012).
Chromium species in soil, solid samples, and pharmaceuticals with IC-ICP/MS according to US EPA 6800
A small amount of chromium is naturally present in soil as a result of its bedrock weathering, but anthropogenic activities can also contribute to the presence of this element in the environment. The prevalent forms of chromium in the environment are Cr(III) and Cr(VI). The opposing properties of these two prevalent species has meant researchers have become increasingly interested in speciation analysis of chromium.
In soil, Cr(III) is mainly adsorbed to macromolecular clay compounds and humic acid, or it occurs in the form of insoluble (hydr)oxides, whereas Cr(VI) occurs in anions (HCrO4- or CrO42-) that are mobile under most conditions. Cr(III) is relatively harmless and is in fact essential to the proper functioning of organisms, but Cr(VI) is a corrosive, acute tissue irritant and carcinogen that is toxic to both animals and plants.
In this analysis, IC-ICP/MS with speciated isotope dilution methodology was used to simultaneously determine soluble Cr(III) and Cr(VI). 53Cr(VI) and 50Cr(III) — isotopically enriched analogues of the analytes — were used to spike the soil. An alkaline solution of ethylenediaminetetraacetic acid (EDTA) was then used to extract the spiked and endogenous analytes in a microwave.
The extracts obtained were examined by the IC-ICP/MS method, and by using the mathematical relationships in speciated isotope dilution mass spectrometry (SIDMS, EPA Method 6800), the separated species were quantified, with simultaneous correction for their method-induced transformations.
It is difficult to directly separate the Cr(III) and Cr(VI) species by chromatography, since they are oppositely charged in solutions. Baseline separation of both species was achieved using a Metrohm IC with the Metrosep A Supp 4 column before analysis was carried out using an Agilent 7700 ICP/MS instrument, as shown in Figure 1. The IC and ICP/MS instruments were synchronized using remote signal.
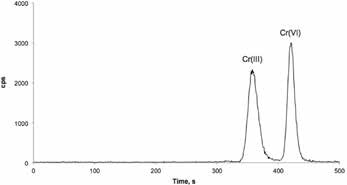
Figure 1. Standard solution containing 10.0 ng/g Cr per species. Column: Metrosep A Supp 4 - 250/4.0; eluent: 2.0 mol/L EDTA (pH 10; isocratic); flow: 0.8 mL/min; injection volume: 100 μL; recording time: 10 min; temperature column off; ICP/ MS parameters: RF power: 1550 W, RF matching: 1.8 V; samplingdepth: 8 mm; plasma gas flow rate: 15 L/min; carrier gas flow rate: 0.95 L/min; makeup gas flow rate: 0.15 L/min; collision gas (He) flow rate: 4.0 mL/min; spray chamber temperature: 2 °C; tuning solution: 1 g/L Li, Co, Y, Ce, and Tl in 2% HNO3 solution; monitoring masses (Cr): 50, 52, and 53 amu; acquisition mode: spectrum and time-resolved analysis (TRA).
Results
It took less than 10 minutes to achieve the baseline separation between Cr(VI) and Cr(III). A number of standard reference materials (SRMs) were analyzed. The measured mass fractions of the Cr(VI) species in the soil SRMs statistically agreed with the certified values at a confidence level (CL) of 95%. The soluble Cr(III) found in all SRMs was less than 3% of the total chromium in the corresponding samples.
Chromate in HCl eluates with Inline Preconcentration, Matrix Elimination, and ICP/MS detection
Hexavalent chromium is toxic and has been categorized as cancerogenic. To ensure consumer safety, consumer goods must be tested for the presence of this compound. In this technique, acidic eluates from inks, plastics¸ chalk, ballpoint pen refills and fabrics were tested for the presence of hexavalent chromium.
Method
1 g of sample was treated with 50 mL of 0.25% HCl, in accordance with DIN EN 71-3, and a buffer was used to neutralize this solution. Next, 2000 μL of this neutralized solution was pre-concentrated and the matrix was automatically removed with water/acetone. Separation was carried out on a Metrosep A Supp 5 – 250/4.0 column and subsequently detected by the ICP/MS method.
In order to prevent the carryover of acetone into the ICP, the column outlet was switched to the ICP/MS just 6 minutes after the injection, and the Cr(VI) peak was detected after 9 minutes. To suppress ArC interference caused by the mobile phase, the mass 52 was captured in collision mode.
Results
This method can be used to quantify Cr(VI) concentrations between 10 and 100 ng/L, as illustrated in Figures 2 and 3. Organic sample components were almost completely removed due to automated elimination of matrix, which enables the analysis of samples with complex matrices and has a positive effect on the column life. This is an extremely sensitive and specific method and requires pure chemicals and a clean working environment, to prevent Cr(VI) blank values (metal spatulas should never be used, for example).
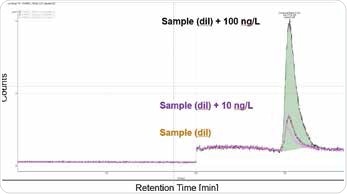
Figure 2. Spiked sample of consumer good
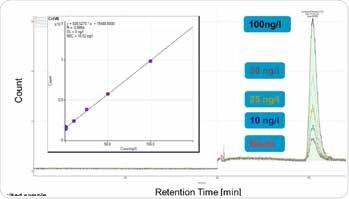
Figure 3. Calibration of hexavalent chromium concentrations between 10 and 100 ng/L. Column: Metrosep A Supp 5 - 250/4.0; eluent: 15.4 mmol/L Na2CO3, 4.8 mmol/L NaHCO3; flow rate: 0.8 mL/min; injection volume: 2000 μL; m/z 52.
Chromium species in dietary supplements using speciated isotope dilution mass spectrometry
To determine the health impact of chromium compound in dietary supplements, it is essential to independently measure and verify Cr(III) and Cr(VI) species by mass balance (sum of the two species must equal independent measurements of total chromium). This is because finished products may contain both species. Since Cr(VI) is stable in alkaline conditions and Cr(III) is stable in acidic conditions, interspecies conversions may take place in complex matrices and also during analytical extraction, which can make quantification more difficult.
A study was performed to determine the presence of Cr(III) and Cr(VI) in dietary supplements. The EPA Method 3060A extraction protocol was carried out to extract Cr(VI) and then EPA Method 3052 was carried out on the extracted residue to digest the Cr(III) remaining.
As illustrated in EPA Method 6800 (update V), speciated isotope dilution mass spectrometry (SIDMS) was implemented with the IC-ICP/MS method. Method 6800 enables the bidirectional chromium interspecies conversions that occur during extraction and sample handling before instrumental analysis, to be tracked and corrected.
Results
Based on mass balance results, the off-the-shelf dietary supplements that were examined contained hexavalent chromium ranging from below the detection limit to 122.4 ± 13.0 μg/g, and this value corresponds to 16% of the total chromium content, as shown in Figure 4. This kind of variation in the final products raises public health concerns and highlights the need for a robust method where speciation can be accurately and reliably analyzed, while correcting for species conversions.
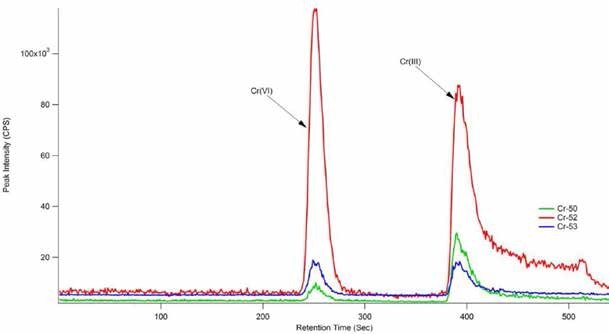
Figure 4. Typical dietary supplement sample. Column: Hamilton PRP-X100 anion-exchange (150 mm × 4.6 mm, 10 μm); eluent A: 0.06 mol/L HNO3, pH = 9.3; eluent B: 0.06 mol/L HNO3, pH 1.2; flow rate: 1.0 mL/min with gradient elution; m/z 50, 52, 53.
Chromate in NIST SRM 2701 by speciated isotope dilution mass spectrometry according to US EPA 6800
The aim of this analysis was to use the EPA Method 6800, which employs SIDMS, and to compare the chromium speciation results obtained from SIDMS against those acquired using conventional methods.
Results
During this study, reproducible and accurate results at very low detection levels for both species of chromium were acquired, as shown in Figure 5. It took less than 15 minutes to analyze each sample but the use of a shorter column can further reduce this time. The Cr(VI) concentration agreed with the reference and certified Cr(VI) concentration for the SRM 2701.
According to NIST’s certificate of analysis for SRM-2701, the true value was determined using Method 6800 SIDMS. SIDMS is unique in its capability to track conversions between species and enable analysts to make legally defensible corrections, particularly for samples with highly reactive matrices such as chromite ore processing residue (COPR). Therefore, the EPA Method 6800 is an indispensable tool for speciation of chromium.
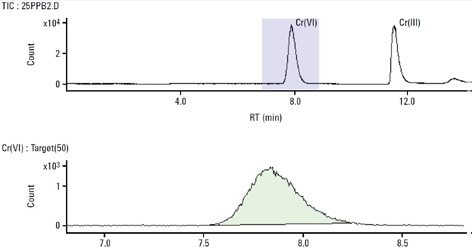
Figure 5. 25 μg/L Cr(III) and Cr(VI) standard. Column: Hamilton PRP-X100 anionexchange (150 mm × 4.6 mm, 10 μm); eluent A: 0.06 mol/L NO3 − (pH 9.3 adjusted with NH4OH); eluent B: 0.06 mol/L NO3 − (pH 1.2); flow rate: 1 mL/min; m/z 50, 52, 53
Speciation of Cr(III) and Cr(VI) by microbore column
The aim of this analysis was to achieve a rapid separation of Cr(VI) and Cr(III) with good resolution of both these species. The column Metrosep Carb 2 - 100/2.0 was used to achieve this.
Results
The two species were isolated as Cr(III)-EDTA complex and chromate and the IC effluent was connected directly to the IPCMS. No flow splitter was required since the flow rate of the fitted microbore column was suitable for the ICP/MS. The chromatographic separation can be completed within 10 minutes, under conditions described in Figure 1.
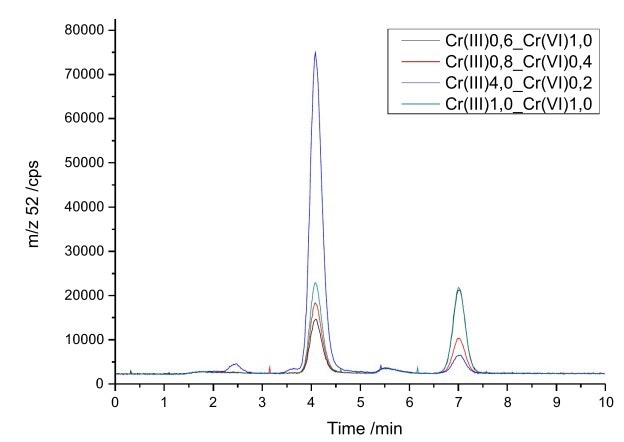
Figure 6. Mixtures of Cr(III) and Cr(VI) with different concentrations (0.2–4.0 μg/L). Column: Metrosep Carb 2 - 100/2.0; eluent c(ammoniumnitrate) = 100 mmol/L, set to pH 9 with ammonia; flow rate 0.2 mL/min.
Chromium – further applications with IC-ICP/MS
- Determination of Cr(VI) in wastewater
- Application Note AN-M-008
- Optimization and validation of strategies for quantifying chromium species in soil based on speciated isotope dilution mass spectrometry with mass balance. Wolle, M.M.; Mizanur Rahman, G. M.; Kingston, H. M.; Pamuku, M. (2014) J. Anal. At. Spectrom. 29(9), 1640–1647 Development of methods for the speciation of chromium in leather waste by IC-ICP/MS. Sushil, K. C. Ph.D. Thesis, Central Ostrobothinia University of applied sciences, Finland, 2010 Use of IC-ICP/MS coupling. Element species analysis of chromium. Knöll, J., Seubert, A. (2009) GIT Labor-Fachzeitschrift 53(3), 151–153
References
- Aschner, M.; Syversen, T. (2005) Methylmercury: Recent advances in the understanding of its neurotoxicity. Ther. Drug Monit. 27, 278–283.
- Bianchi, A.; Calabi, L.; Corana, F.; Fontana, S.; Losi, Maiocchi, A.; Paleari, L.; Valtancoli, B. (2000) Thermodynamic and structural properties of Gd(III) complexes with polyaminopolycarboxylic ligands: Basic compounds for the development of MRI contrast agents. Coord. Chem. Rev. 204, 309–393
- Burger, J.; Gochfeld, M. (2005) Heavy metals in commercial fish in New Jersey. Environ. Res. 99(3), 403–412
- Dietary Supplement Fact Sheet: Selenium. US National Institutes of Health; Office of Dietary Supplements. Retrieved Oct 11, 2016.
- Gochfeld, M.; Burger, J. (2005) Good fish/bad fish: A composite benefit–risk by dose curve. Neurotoxicology 26(4), 511–520
- Herrmann, T. Einsatz der On-line-Kopplung von Ionenchromatographie und ICP-MS zur Bestimmung von Anionen. Diploma thesis, Philipps-Universität Marburg, Germany, 2006
- Knöll, J. Ph.D. Ultratrace determination of aminopolycarboxylic acid based chelating agents using inverse on-line coupling of IC with ICP-MS. PhD thesis, Philipps-Universität Marburg, Germany, 2013
- Knöll, J.; Seubert, A. (2012) Indirect ultra trace determination of aminopolycarboxylic acids in surface water using ion exchange chromatography coupled on-line to inductively coupled plasma mass spectrometry. Journal of Chromatography A 1270, 219-224.
- Kümmerer, K.; Ed. Pharmaceuticals in the environment: Sources, fate, effects, and risks. Springer-Verlag Berlin Heidelberg, 2008; 3rd ed.
- Künnemeyer, J.; Terborg, L.; Meermann, B.; Brauckmann, C.; Möller, I.; Scheffer, A.; Karst, U. (2009) Speciation analysis of gadolinium chelates in hospital effluents and wastewater treatment plant sewage by a novel HILIC/ICP-MS method. Environ. Sci. Technol. 43(8), 2884–2890.
- Künnemeyer, J.; Terborg, L.; Nowak, S.; Telgmann, L.; Tokmak, F.; Krämer, B. K.; Günsel, A.; Wiesmüller, G. A.; Waldeck, J.; Bremer, C.; Karst, U. (2009) Analysis of the contrast agent Magnevist and its transmetalation products in blood plasma by capillary electrophoresis / electrospray ionization time-of-flight mass spectrometry. Anal. Chem. 81(9), 3600–3607
- Levenson, C. W.; Axelrad, D. M. (2006) Too much of a good thing? Update on fish consumption and mercury exposure Nutr. Rev. 64(3), 139–145.
- Montaser, A.; Golightly, D. W.; Eds. Inductively coupled plasmas in analytical atomic spectrometry. VCH Publishers, Inc.: New York, 1992.
- Rahman, G. M. M.; Martone, N.; 'Skip' Kingston, H. M. (2012) Determination of hexavalent chromium in NIST SRM 2701 by speciated isotope dilution mass spectrometry (EPA Method 6800) using IC-ICP-MS. In Handbook of hyphenated ICP-MS applications, 2nd edition; Agilent, 2012; pp 33–35. http://www.agilent.com/cs/library/applications/5990-9473EN_icpmsSpeciationHB_lr.pdf (accessed Oct 7, 2016)
- Saranko, C. J. et al. Fact Report for toxicity of arsenite and arsenate, Florida Dept. of Health, Nov 6, 1998
- Schedlbauer, O. F.; Heumann, K. G. (2000) Biomethylation of thallium by bacteria and first determination of biogenic dimethylthallium in the ocean. Appl. Organomet. Chem.14(6), 330-340
- Wilber, C. G. (1980). Toxicology of selenium: A review.Clin. Toxicol. 17 (2), 171–230.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.