Maintaining high levels of drug quality and safety is priority for the pharmaceutical industry. Pharmacopoeias have documented these standards as officially acknowledged pharmaceutical rules. Government and medical societies have also published these rules as legal tools for protecting customers.
The devices and methods used for drug identification and its compliance with regulatory standards need to be reliable and sensitive. The most preferred method to analyze different types of pharmaceutical solutions, pharmaceuticals, or even body fluids for determining the active ingredients, trace impurities, excipients and metabolites occurring as organic or inorganic ions or polar substances, is ion chromatography (IC).
Using ion chromatography, several substances can be detected in a single analysis rapidly, and chemically similar analytes can also be differentiated. The range of analyte concentration that can be measured by this method varies from ng/L to percentages.
Another advantage of the IC method is the availability of a wide range of elution systems and separation columns that enable analysis of virtually all types of analytes. Moreover, selecting an appropriate detection method or sample preparation method helps eliminate the interfering effects of the sample matrix.
Most of the existing IC systems feature inline sample preparation for convenient operation. Besides simplifying the IC method, automation also alleviates the risk of contamination and erroneous results considerably by minimizing human intervention. Selecting an appropriate detection method is based on the specifications of the analyte and matrix:
- Electrochemical detection
- Conductivity detection both with and without suppression
- Spectrometric detection both with and without post-column derivatization, for instance, UV-visible spectrophotometry
- Coupled detection techniques like IC-mass spectrometry (MS) and IC-inductively coupled plasma-MS
Different ion chromatographic methods are required for different forms of pharmaceutical samples. This article provides an overview of the common sample types and corresponding analysis methods. There are over 80 IC applications in the pharmaceutical industry, of which three applications are presented in Table 1.
Table 1. Selection of IC applications in the pharmaceutical industry
|
Pharmaceutical
|
Analyte
|
Detection
|
|
Adrenaline
|
Adrenaline
|
API
|
Amperometric detection
|
|
Ibuprofen
|
Ibuprofen, Valerophenone
|
API
|
Spectrophotometric detection
|
|
Zoledronate/ Zoledronic acid
|
Phosphite, phosphate
|
Impurities
|
Conductivity detection
|
Pharmaceutical Solutions
Pharmaceutical solutions such as infusion, hemodialysis, and isotonic solutions consist of organic acids, carbohydrates, anions, and cations, with frequently differing concentrations from one another on the order of several magnitudes.
It is necessary to have a rapid and easy-to-operate analysis techniques to accurately determine these ingredients for monitoring production efficiency and final quality control. IC is an ideal method to meet these requirements, thanks to its automatic inline sample preparation and intelligent analytical procedure. Figures 1 and 2 illustrate the analysis of hemodialysis solutions.
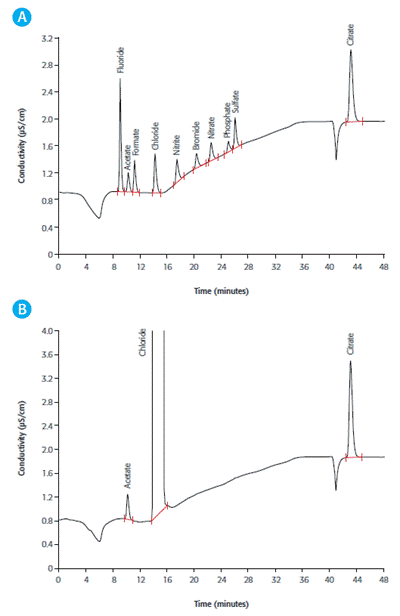
Figure 1. IC measurement on a Metrosep A Supp 7 - 250/4.0 using Na2CO3 gradient elution, followed by sequential suppression and conductivity detection A: Anion standard including acetate and citrate B: Acetate and citrate in hemodialysis solution
Hemodialysis needs to be performed for patients having renal failure to make up for the loss of the blood-cleansing function of their kidney. It involves exchanging solutes between a hemodialysis solution and the patient’s blood via a semi-permeable membrane.
As part of the process, waste products such as phosphate and urea are also diffused into the dialysis solution. Dialysis solutions have a complex composition because the osmotic activity of the blood changes when solutes are removed from it. Therefore, having an appropriate solution concentration is essential for the process to be happened in a controlled rate.
Dialysis disequilibrium syndrome can occur when the osmotic activity changes strongly, causing the removal of solutes from other body compartments because of the low solute concentration in the blood. Acetate and citrate in diluted hemodialysis solution are simultaneously determined in Figure 1.
The measurement of an anion standard is shown in part A and the sample determination is depicted in part B. Hemodialysis solutions contain acetate as a buffer substance and citrate for its anticoagulant properties. Their presence helps stabilize the pH value of the blood of dialysis patients, whose kidneys fail to excrete acid components.
From the chromatogram, it can be observed that chloride is present in near-physiological concentration. Reducing the concentration gradient to a minimum by applying physiological solute concentrations helps achieve a dynamic equilibrium between the dialysis solution and blood, thereby preventing the loss of solutes such as chloride.
Cation determination in hemodialysis concentrate subsequent to an automated inline dilution step is presented in Figure 2. The removal of cations from the blood by osmosis can be avoided when they are present in near-physiological concentrations.
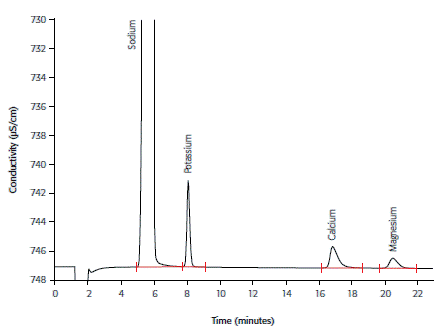
Figure 2. Cations in diluted hemodialysis concentrate using the Metrosep C 4 - 150/4.0 column and non-suppressed conductivity detection
Active Pharmaceutical Ingredients (APIs)
IC can determine the presence of APIs such as bethanechol chloride, cefadroxil, neomycin, and gentamicin in drugs as per the requirements of the US Pharmacopeia and the European Pharmacopoeia. The IC analysis results for a bactericidal antibiotic called gentamicin are depicted in Figure 3.
Bacterial antibiotics bind to ribosomes to cause errors in the translation from mRNA to DNA, and as a result, bacterial protein biosynthesis is blocked. Gentamicin C1, C1a, C2, C2a, and C2b are the other forms of gentamicin with similar structure. However, they can be effectively distinguished from one another with IC.
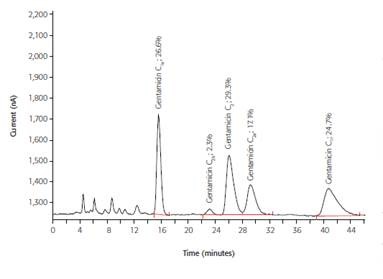
Figure 3. IC determination of the antibiotic gentamicin by pulsed amperometric detection; column: Polymer Laboratories RP-S; eluent: 60g/L Na2SO4, 1.75g/L sodium octane sulfonate, 1.34g/L NaH2PO4, 8mL/L THF (pH = 3, H3PO4); post-column addition: 300mmol/L NaOH
Impurities in Pharmaceutical Ingredients
IC can also determine whether the pharmaceutical products have impurities, which can lead to adverse side effects even in their trace quantities. For instance, azide is a strongly toxic impurity to humans that can be found in trace amounts in the synthesis of the antihypertensive irbesartan.
Therefore, stringent control measures have to be in place in irbesartan production. Ion chromatographic azide determination subsequent to direct injection as outlined in USP 621 is recommended by the US Pharmacopeia. The method involves API removal from the analytical column using a transfer solution composed of an appropriate organic solvent and IC eluent. Nevertheless, this method is laborious, time intensive and not suitable for automation.
Conversely, using inline matrix elimination enables rapid, more-sensitive and more-selective azide determination by separating the interfering pharmaceutical matrix from the target analyte during sample preparation. The IC analysis results of an irbesartan sample that was spiked with varying azide concentrations are presented in Figure 4.
A conductivity detector records the signal subsequent to sequential suppression. The mean conductivity determined by the detector and the relative standard deviation, as well as the average recovery results of the azide obtained from three measurements are summarized in Table 2.
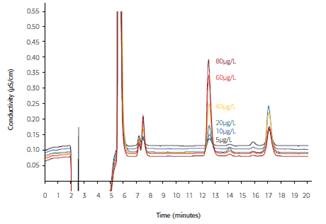
Figure 4. Irbesartan sample spiked with 5-80μg/L azide; column: Metrosep A Supp 10 - 250/4.0; eluent: 5mmol/L Na2CO3, 5mmol/L NaHCO3; inline matrix elimination with 70:30 (v/v) methanol/water
Table 2. Precision and recovery of azide
|
|
Peak area
|
|
|
|
Mean value (μS/cm)
|
Relative standard deviation (%)
|
Recovery (%)
|
|
5μg/L spike
|
0.4223
|
1.96
|
101.71
|
|
30μg/L
|
spike
|
2.5754
|
0.14
|
|
n=3 measurements
|
The use of inline matrix elimination for determining azide content in irbesartan meets all prerequisites of the regulatory authorities pertaining to the method’s accuracy, linearity, robustness, selectivity, and limits of detection and quantitation, making it a rapid and more-sensitive alternative to the detection method recommended by USP 621.
Radio IC
Radiopharmaceuticals are radioactive substances that can be analyzed for their radiochemical purity using radio IC. Besides being used in medical purposes such as diagnostics, radiopharmaceuticals are also used for treating and preventing certain diseases. Radiotracers such as [18F] fluorocholine and [18F] fluorodeoxyglucose are widely used in positron emission tomography diagnosis, and are labeled with the radionuclide [18F] fluorine. This unstable isotope emits a positron and a neutrino during its radioactive decay involving the conversion of a proton in the nucleus of [18F] fluorine into neutron. The positron couples to an electron in the adjacent tissue, causing annihilation of both particles. Consequently, two photons (gamma rays), each with 0.511MeV energy, are emitted in opposite directions.
Coincidence detection of the photon pair helps calculate the location of emission of the pair in the patient body, which is in close coincidence with the position of the original radiotracer molecule. This, in turn, provides data pertaining to its activity. Radiotracers used must be free from contamination because highly energetic gamma rays released by positron-electron coupling could be adverse to the human body. The use of a pure radiotracer keeps the level of radioactive substance injected to the patient to a minimum by eliminating the introduction of radioactive contaminants such as free [18F] fluorine.
Radio IC establishes the QC of radiotracers in the short time between their production and capturing of the 3D PET scan. Like traditional IC, radio IC has the same separation step but it occurs behind lead doors. However, its detection step is completely different from the conventional IC, involving a radioactivity detector. The presence or absence of radioactive contaminants can be clearly determined from the radioactivity chromatogram.
Conclusion
In the pharmaceutical industry, IC is used in a number of applications. The availability of a wide choice of columns, sample preparation techniques, gradient and eluent options, and automation capabilities makes IC a highly versatile method.
Acknowledgements
Produced from materials authored by Stephanie Kappes, Alfred Steinbach and Katinka Ruthfrom from Metrohm AG, CH-9101 Herisau/Switzerland.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.