Although the known photometric techniques for the calculation of ammonia are accurate, they take up a substantial amount of time (Nessler technique 30 min, indophenol technique 90 min reaction time). Another drawback of these techniques is that only clear solutions can be quantified. Opaque solutions must be clarified by time-consuming procedures first.
With the ion-selective ammonia electrode, these problems do not exist. Measurements can be carried out easily in liquid fertilizer, waste water, and urine in addition to soil extracts. Particularly for waste water and fresh water samples, a number of standards such as ASTM D1426, ISO 6778, EPA 350.2, and EPA 305.3, outline the analysis of ammonium by ion measurement.
In this article, the determination according to these standards is outlined besides the ascertainment of other samples, in addition to some general tips and tricks on how to handle the ammonia ion selective electrode.
Calculation of the nitric acid content in nitrates, of ammonia in ammonium salts, and of the nitrogen content of organic compounds with the ion-selective ammonia electrode is based on the principle that the ammonium ion is released as ammonia gas upon addition of excess caustic soda: NH4+ +OH- = NH3 + H2O.
The outer membrane of the electrode permits the ammonia to diffuse through. The alteration in the pH value of the inner electrolyte solution is observed using a combined glass electrode. If the substance to be measured is not present in the form of an ammonium salt, it must be converted into one first.
Organic nitrogen compounds, particularly amino compounds are digested according to Kjeldahl by heating with concentrated sulfuric acid. In the process, the carbon is oxidized to carbon dioxide while the organic nitrogen is changed quantitatively into ammonium sulfate.
Instruments
- Stirrer
- 10 mL buret (for standard addition)
- Titrator with ‘STDADD’ and ‘MEAS Conc’ mode
Electrode
| . |
. |
NH3-selective electrode
(for low concentration) |
6.0506.100 |
NH3-selective electrode
(for high concentration) |
6.0506.150 |
General Hints
Handling of the Electrode
To obtain optimal measurements, the following points should be observed:
- The inner glass electrode must be soaked for 6 to 12 hours in an ammonia - free pH = 4 buffer solution after a longer storage in the dry state or before the first use. Afterwards, rinse with distilled water and dry by dabbing with a soft paper towel.
- As outlined in the manual (8.109.8031) both electrodes should be mounted with the membrane. 2.5 mL of outer electrolyte solution is added. After it is mounted together, the electrode must be carefully shaken to remove any air bubbles from the membrane. Then for approximately 10 minutes the electrode is placed in distilled water.
- The membrane of the electrodes must never be touched with your fingers or any kind of objects, as this would decrease the water-repellant effect of the membrane.
- After each measurement, rinse the electrode with distilled water and remove any adhering water droplets by dabbing with a soft paper towel. The electrode should be conditioned in distilled water for at least 10 min before each new measurement. For calculating concentrations which are less than 1 mg/L, the electrolyte must be diluted 1:9 with deionized water and the electrode must be conditioned for approximately 30 min. The electrode is placed in c(NH4CI) = 0.1 mol/L for storage times of 1 to 5 days. Take the electrode apart for longer periods, clean the outer shaft with deionized water and store the inner part (pH electrode) in KCl sat.
- A too large slope in a standard addition is typically because of too short conditioning times or an error in the electrode preparation. Yet, slopes which are not over 3 mV above the theoretical value can be tolerated.
- The measurements should be carried out at a constant temperature using a constant stirring speed. Stirring should be continuous as bubbles can form on the membrane during an interruption in stirring, which can lead to false readings. Ensure that the stirrer is switched on and that a stirring bar is in the measuring vessel. Erroneous results are gathered if additions are made without stirring.
- The membrane and inner electrolyte must be replaced if more than five to six minutes are required to gather a stable reading.
- If samples with largely different ammonia concentrations are frequently measured, the time needed to attain a stable reading becomes longer for the samples with lower ammonia concentrations.
- Refer to the hints given in the manual (8.109.8031) for NH3-selective electrodes.
Possible Interferences
- Hydrazine:
- Causes the same interference as outlined above for volatile amines.
- Complexing ions:
- Cu, Ag, Hg, and Ni among others simulate a lower ammonia concentration. The interference caused by 20 ppm Hg can be stopped by utilizing a solution with an additional 0.46 g Nal per 100 mL NaOH instead of the NaOH solution mentioned.
- Volatile amines:
- Simulate an elevated concentration of ammonia. Ethylene amine gives almost the same difference in potential as ammonia. Longer C chains cause lower interference.Proteins:
- A high protein concentration (1 g/L) simulates bigger ammonia concentrations. The calibration curve becomes linear only above 10 mg/L of ammonium.
- Concentration:
- Should not be over 1 mol/L. The sample must be diluted with distilled water at higher concentrations. Yet, in lower concentration ranges, the electrode becomes less sensitive and needs more time to reach a stable reading (up to 15 min).
Sample Preparation and Handling
- The sample must be acidulated with hydrochloric acid to approx. pH 6 (approx. 0.5 mL c(HCl) = 1 mol/L per liter of sample) for the purpose of storage, and kept in well-sealed vessels.
- All measurements should be carried out at a constant temperature (for example, at 25 °C). Standard and sample solutions should always be measured at the same temperature and the same stirring speed. A change in temperature of 1 °C results in a deviation of approx. 2%.
- Do not add the sodium hydroxide solution until just before the measurement.
- The pH value must be at least 12 after the addition of NaOH. Acidic samples must be neutralized before the addition of NaOH. The measurement must take place immediately after addition of NaOH, otherwise the ammonia evaporates and leads to too low results.
- The ammonia loss in a stirred alkali sample of 100 mL is approx. 50% within six hours. Alkali samples must be measured immediately.
- Ammonia dissipates extremely quickly out of solution when concentrations are > 1 mol/L. Therefore, these kinds of samples must be diluted.
- If a standard or sample solution is measured repeatedly, then it must be stored in a sealed or covered vessel between the measurements. This ensures that ammonia will not escape. It is recommended that the standard be prepared right before the measurement directly in the beaker for best practice.
- Utilize narrow, high measuring vessels (minimum ratio of surface to volume) where possible.
Choice of Procedure
- When several measurements of the same solutions are to be performed in a defined and unproblematic sample matrix, direct measurement is preferred.
- Standard addition is a fast and reliable technique, particularly for undefined sample matrices.
Choice of Standard Concentrations
- To ensure an accurate evaluation of the standard addition, the standard concentrations (cstd) for the different buret volumes (Vburet / mL) must be chosen as a function of the sample concentration (csmp1) according to the table below:
| Vburet / mL |
Cstd/ Csmpl |
| 5 |
40:1 |
| 10 |
20:1 |
| 20 |
10:1 |
| 50 |
5:1 |
If the sample has been substantially diluted with ISA or TISAB, this ratio must be considered.
| . |
. |
. |
| Sample concentration (csmpl) |
5 |
mg/L |
| Buret volume (Vburet) |
10 |
mL |
| Sample size |
10 |
mL |
| ISA/TISAB |
10 |
mL |
| Total volume (Vtotal) |
20 |
mL |
| Factor from table (cstd : csmpl) |
20 |
|
The result is a sample concentration of 2.5 mg/L in the initial solution. The optimal concentration of the standard is therefore 2.5 mg/L x 20 = 50 mg/L.
- However, note that this is just a general guide for the standard concentration. Exact measurements are also viable if the above recommendations are not followed strictly.
- Low ammonia concentrations: The adjustment time becomes distinctly longer. The blank values of the chemicals must be considered.
- The standard solutions must have the same ionic background as the sample solutions.
Direct Measurement
Direct measurement is a simple and rapid method for testing numerous samples with similar sample matrices across a wide range of concentrations. Observe the following notes when performing direct measurements:
- Ensure that the temperatures of all sample and standard solutions are identical.
- Choose the concentrations of the standard solutions (e.g, NH4Cl solutions) in such a way that the expected ammonia concentration of the sample solution falls in the middle of the calibration range.
- The diffusion of ammonia through the membrane is considerably slowed down in the presence of ammonia concentrations < 6 – 10-5 mol/L. The response time of the electrode is prolonged accordingly. A special measurement method is needed for this concentration range.
Calibration of High Ammonia Concentrations and Analysis of Waste Water Samples
Determination according to ISO 6778 is described in this section.
Reagents
- Sodium hydroxide
- Disodium ethylenediaminetetraacetate, EDTA
- Metrohm measuring electrolyte (50 mL); 6.2316.030
- Ammonium chloride
Solutions
| . |
. |
| ISA solution |
c(NaOH) = 1 mol/L and c(EDTA) = 0.1 mol/L
40 g NaOH and 37.2 g EDTA are weighed into a 1 L volumetric flask containing approx. 800 mL distilled water. After dissolving and reaching room temperature, the solution is filled up to the mark with deionized water. |
Standard Solutions
| . |
. |
| 10’000 mg/L ammonium |
29.7 g ammonium chloride is weighed into a 1 L volumetric flask, dissolved in deionized water and filled up to the mark with deionized water. |
| 10 mg/L ammonium |
1 mL β(NH4) = 10’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
| 50 mg/L ammonium |
5 mL β(NH4) = 10’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
| 90 mg/L ammonium |
9 mL β(NH4) = 10’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
| 130 mg/L ammonium |
13 mL β(NH4) = 10’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
| 170 mg/L ammonium |
17 mL β(NH4) = 10’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
Calibration
50 mL of standard is pipetted into a plastic beaker and 5 mL ISA solution is added for each standard concentration. The standards are measured in ascending order, by concentration. The sensor is rinsed with distilled water and conditioned in distilled water for exactly 10 min before the measurement of the first standard and in-between measurements of the different standards.
Analysis
50 mL of waste water is pipetted into a plastic beaker, then 5 mL ISA solution is added, and the measurement begins. The sensor is rinsed with distilled water and then conditioned in distilled water for exactly 10 minutes between measurements.
Parameters (Calibration and Analysis)
| . |
. |
| Number of standards |
5 |
| Unit Conc. |
ppm |
| Conc. standard 1 |
10 |
| Conc. standard 2 |
50 |
| Conc. standard 3 |
90 |
| Conc. standard 4 |
130 |
| Conc. standard 5 |
170 |
| Stirring rate |
8 |
| Signal drift |
0.50 mV/min |
| Min. waiting time |
10 s |
| Max. waiting time |
600 s |
| Measuring interval |
2.0 s |
Example Calibration Curve
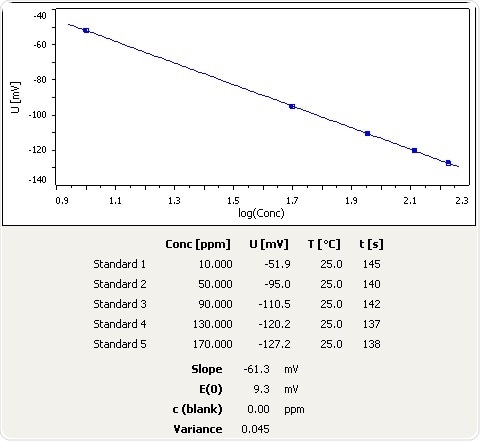
Figure 1: Calibration curve for NH3 selective electrode.
Example Sample Curve
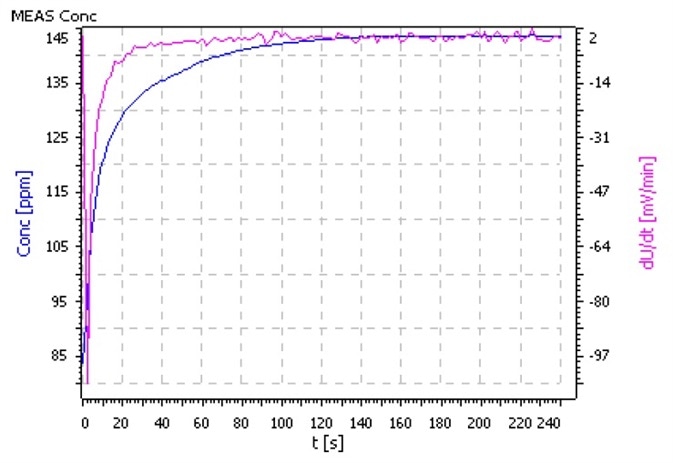
Comments
- Utilize as big a sample volume as possible so that the ratio of surface to volume is as low as it can be. This will minimize the absorption of ammonia from ambient air.
- Condition the electrode prior to measurement in an ammonia – free pH 4 buffer solution.
Calibration of Low Ammonia Concentration and Analysis of Tap Water
The calculation which is outlined here is based on ASTM D1426 and EPA 350.3. The volume of sample was reduced compared to the two standards.
Reagents
- Sodium hydroxide
- Metrohm measuring electrolyte (50 mL); 6.2316.030
- Ammonium chloride
Solutions
| . |
. |
| ISA solution |
c(NaOH) = 10 mol/L
200 g ice made from distilled water and 400 mL distilled water are placed into a plastic beaker and 400 g NaOH is added. After dissolving and reaching room temperature, the solution is transferred into a 1 L volumetric flask and filled up to the mark with deionized water. |
| Diluted electrolyte |
The Metrohm measuring electrolyte 6.2316.030 is diluted 1:9 with deionized water. |
Standard Solutions
| . |
. |
| 1’000 mg/L ammonium |
2.97 g ammonium chloride is weighed into a 1 L volumetric flask, dissolved in deionized water and filled up to the mark with deionized water. |
| 0.1 mg/L ammonium |
0.1 mL β(NH4) = 1’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
| 0.5 mg/L ammonium |
0.5 mL β(NH4) = 1’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
| 0.9 mg/L ammonium |
0.9 mL β(NH4) = 1’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
| 1.3 mg/L ammonium |
1.3 mL β(NH4) = 1’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
| 1.7 mg/L ammonium |
1.7 mL β(NH4) = 1’000 mg/L is pipetted into a 1 L volumetric flask and filled up to the mark with deionized water. |
Calibration
20 mL of standard is pipetted into a plastic beaker and 1 mL ISA solution is added for each standard concentration. The standards are measured in ascending order by concentration. The sensor is rinsed with distilled water and conditioned in distilled water for exactly 10 minutes before the measurement of the first standard and between the measurement of the different standards.
Analysis
The measurement begins after 20 mL of tap water is pipetted into a plastic beaker and 1 mL ISA solution is added. The sensor is rinsed with distilled water and then conditioned in distilled water for exactly 10 minutes between measurements.
Parameters (Calibration and Analysis)
| . |
. |
| Number of standards |
5 |
| Unit Conc. |
ppm |
| Conc. standard 1 |
0.1 |
| Conc. standard 2 |
0.5 |
| Conc. standard 3 |
0.9 |
| Conc. standard 4 |
1.3 |
| Conc. standard 5 |
1.7 |
| Stirring rate |
8 |
| Signal drift |
0.50 mV/min |
| Min. waiting time |
10 s |
| Max. waiting time |
600 s |
| Measuring interval |
2.0 s |
Example Calibration Curve
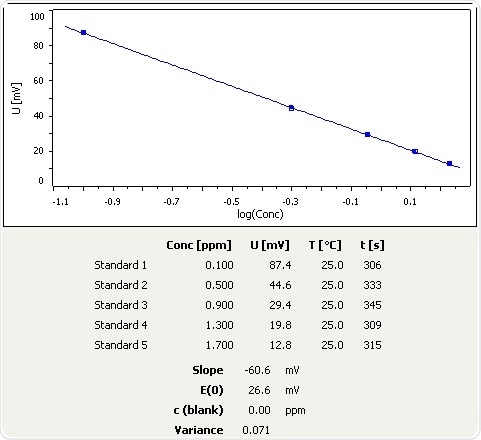
Figure 2: Calibration curve for NH3 selective electrode in the low calibration range.
Example Sample Curve
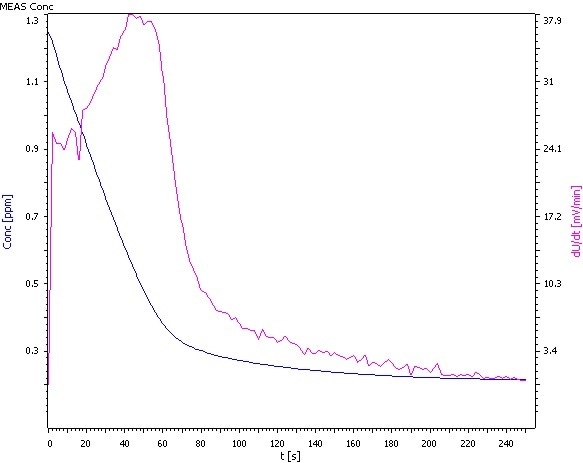
Figure 3: Analysis of β(NH4) = 0.25 mg/L.
Comments
- The standards ASTM D1426, EPA 350.2, and EPA 350.3 are different from the procedure outlined here, in such a way that 100 mL samples are utilized instead of the 20 mL described.
- At this low concentration range, it is crucial to prepare the standards as shortly as possible before measurement. If not, the calibration curve will not be linear, and the measurement becomes faulty.
- The electrolyte of the outer compartment is diluted 1:9 with deionized water if low concentrations of ammonia are measured.
- Condition the electrode in an ammonia – free pH 4 buffer solution before measurement.
Standard Addition
There are three kinds of standard addition in principle:
- man (Standard addition with manual addition of standard addition solution.)
- dos (Standard addition with addition of standard addition solution by a dosing device.)
- auto (Standard addition with automatic addition of standard addition solution from a dosing device in such a way that a constant potential difference results.)
For the characteristics of the individual types, see the manual of your device or tiamoTM. The most reliable technique is automatic standard addition (auto). However, note that the difference in potential ΔU selected must be at least 12 mV per standard addition and that at least four standard additions must be carried out (total ΔU at least 30 mV) for accurate results. If exactly defined volumes of the standard additions are needed but maximum ease of operation is also desired, select ‘auto dos’ and enter the individual standard addition volumes.
Reagents
| . |
. |
ISA solution
c(NaOH) = 10 mol/L |
400 g NaOH is dissolved in approx. 500 mL deionized water containing ice cubes made of deionized water. After dissolution, the solution is transferred into a 1 L volumetric flask and filled up to the mark with deionized water. |
EDTA solution
c(EDTA) = 1 mol/L |
29.22 g EDTA is suspended in 50 mL water and c(NaOH) = 10 mol/L is added dropwise until everything was dissolved. Afterwards the solution is transferred into a 100 mL volumetric flask and filled up to the mark with deionized water |
Standard Solutions
| . |
. |
| 10’000 mg/L ammonium |
29.7 g NH4Cl is weighed into a 1 L volumetric flask, dissolved and filled up to the mark with deionized water. |
| 1000 mg/L ammonium |
2.97 g NH4Cl is weighed into a 1 L volumetric flask, dissolved and filled up to the mark with deionized water. |
| Standard c(NH4) = 0.100 g/L ~ 100 ppm |
0.297 g NH4Cl is weighed into a 1 L volumetric flask, dissolved and filled up to the mark with deionized water. |
| Standard c(NH4) = 0.010 g/L ~ 10 ppm |
10 mL of c(NH4) = 1’000 mg/L is transferred into a 1 L volumetric flask and filled up to the mark with deionized water. |
Sample Preparation
For tap water, melted snow, aquarium water, and liquid fertilizer, sample preparation is not required.
Soil
Approx. 30 g dried soil (2 h at 120 °C) is weighed into a 500 mL beaker and 300 mL c(HCl) = 2 mol/L is added. The suspension is boiled for 1 hour and allowed to cool down. Next the suspension is filtrated into a 500 mL volumetric flask and filled up to the mark with deionized water.
Analysis
Soil
A 0.5 mL prepared soil solution is pipetted into a 100 mL beaker and 49.5 mL water plus 1 mL c(EDTA) = 1 mol/L is added. The standard addition with β(NH4) = 100 mg/L is started immediately after the addition of 1 mL c(NaOH) = 10 mol/L.
Tap water and Melted Snow
A 50 mL sample is pipetted into a 100 mL beaker and 1 mL c(NaOH) = 10 mol/L is added. The standard addition with β(NH4) = 10 mg/L begins immediately afterwards.
Fertilizer
0.1 mL fertilizer, 49.9 mL deion water and 1 mL c(EDTA) = 1 mol/L are pipetted into a 100 mL beaker and 1 mL c(NaOH) = 10 mol/L is added. The standard addition with β(NH4 = 10’000 mg/L is started directly after the addition of the c(NaOH) = 10 mol/L.
Aquarium Water and Mineral Water
50 mL sample is pipetted into a 100 mL beaker and 1 mL c(EDTA) = 1 mol/L is added. The standard addition with β(NH4) = 10 mg/L is started immediately afterwards.
Parameters
STDADD Auto
| . |
. |
| Stirring rate |
6 |
| Number of additions |
4 |
| Volume auxiliary solution |
1 mL (for tap water, aquarium water)
51.9 mL (for fertilizer)
51.5 mL (for soil) |
| Stop volume |
10 mL |
| Dosing rate |
fast |
| Delta U |
12 mV |
| Signal drift |
0.5 mV/min |
| Min. waiting time |
60 s |
| Max. waiting time |
300 s |
| Measuring interval |
2.0 s |
| Switch off stirrer during measurement |
off |
Calculation
No additional calculation is required for aquarium water, mineral water, tap water, and melted snow.
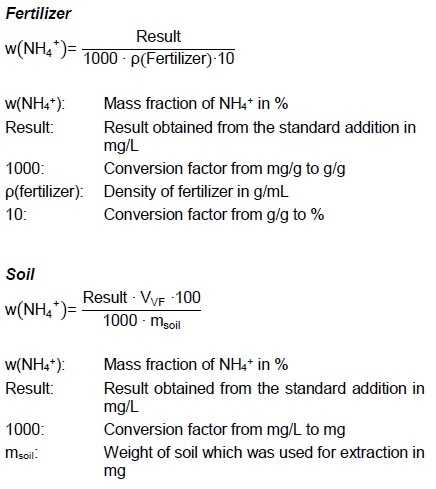
Example Curves
Tap Water
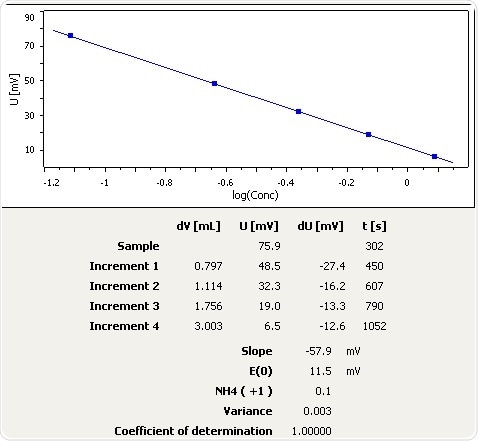
Figure 4: Graph of the standard addition of ammonia in tap water.
Aquarium Water (Sea Water)
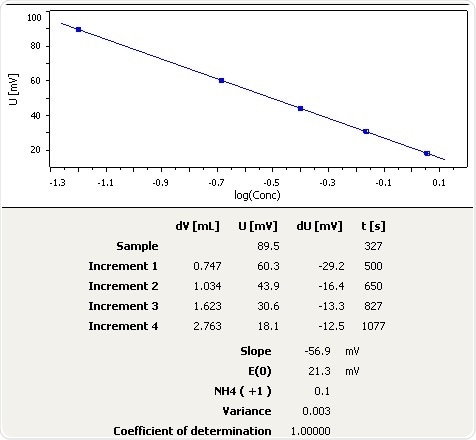
Figure 5: Graph of the standard addition of ammonia in aquarium water (seawater aquarium).
Melted Snow
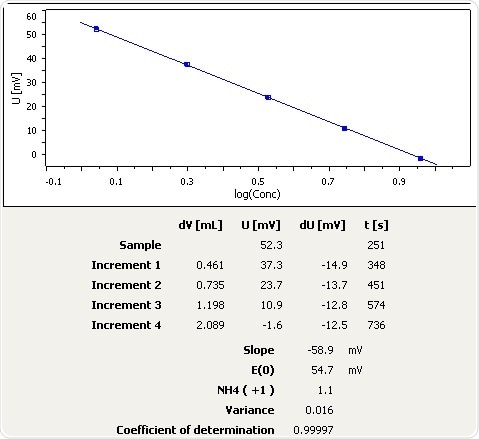
Figure 6: Graph of the standard addition of ammonia in melted snow.
Mineral Water
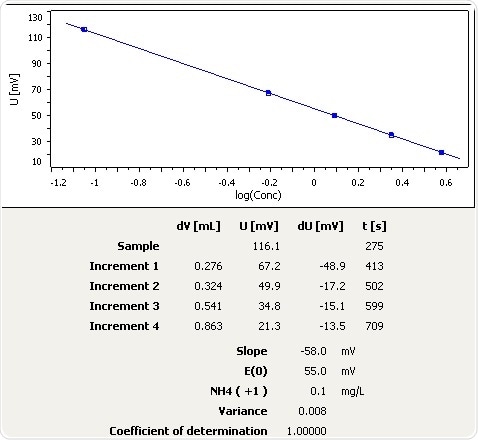
Figure 7: Graph of the standard addition of ammonia in mineral water.
Fertilizer
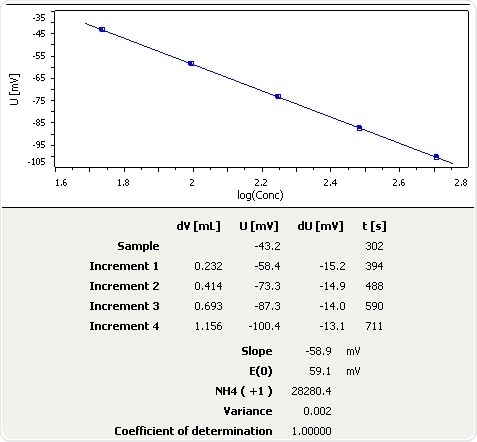
Figure 8: Graph of the standard addition of ammonia in fertilizer.
Soil
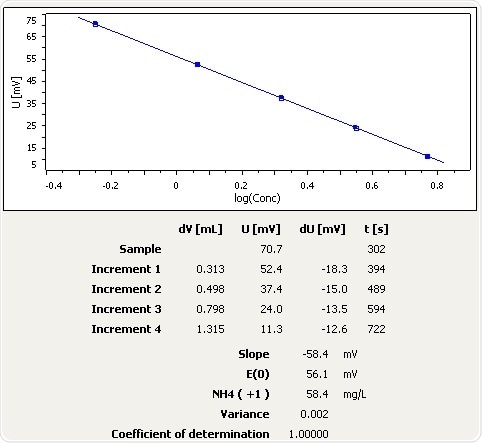
Figure 9: Graph of the standard addition of ammonia in soil.
Comments
- For low ammonia concentrations, the electrolyte is diluted with deionized water 1:9.
- After the addition of c(NaOH) = 10 mol/L, the stabilization of the measuring signal takes some time. Hence, it is necessary to increase the minimum waiting time to one minute.
- The electrode is conditioned for 10 minutes in ammonia – free buffer pH 4 prior and between measurements.
References
- EPA 350.2 Nitrogen, ammonia (Colorimetric, titrimetric, potentiometric - distillation procedure)
- EPA 350.3 Nitrogen, ammonia (Potentiometric, ion selective electrode)
- ASTM D1426 Standard Test Methods for Ammonia Nitrogen In Water
- ISO 6778 Water quality - Determination of ammonium. Potentiometric method
- Manual NH3-selective electrodes (8.109.8031)
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.