One of the most commonly used varieties of plastic are polyurethanes. They are created from the reaction of raw polyols with isocyanates. A large variety of plastics can be acquired depending on the starting material. Establishing the hydroxyl value, acid value, and isocyanate content plays a key role in the analysis of raw materials for plastics.
To ensure batch-to-batch uniformity, the acid number of polyol raw material is typically utilized in quality control. In addition, it is employed as a correction factor for determining the true hydroxyl number. In this article, the determination of the acid number according to ASTM D4662 and ASTM D7253 is outlined.
Polyols are one raw material used for polyurethanes and are made up of multiple hydroxyl groups. Hence, the hydroxyl number of a raw material corresponds directly to the amount of polyols present, and so it is a crucial parameter for quality control. In this article, the calculation of the hydroxyl number according to ASTM E1899 and DIN 53240-3 is outlined.
The knowledge of the isocyanate content is an important quality parameter for the production of polyurethanes, as polyols react stoichiometrically with isocyanates. The determination according to EN ISO 14896 method A, ASTM D5155 method A and ASTM D2572 is described below.
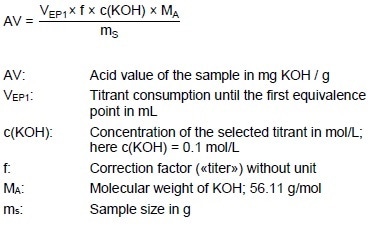
Acid Value (AV)
Summary
The acid value corresponds to the amount of carboxylic acid groups in alkyl resins, polyester acrylate resins or mixtures and is stated in mg KOH per g sample. It is also utilized in evaluating plasticizers, where acid values should be as low as possible.
Instruments
- Stirrer Electrodes
- Titrator with DET mode
- Sample changer
- Buret 20 mL
Electrodes
| . |
. |
| Solvotrode easyClean |
6.0229.020 |
Reagents
- Toluene, dry
- Ethanol
- Phenolphthalein
Solutions
| . |
. |
| Titrant |
c(KOH) = 0.1 mol/L in ethanol or methanol
If possible this solution should be bought from a supplier. |
| Solvent mixture |
Ethanol / toluene, Φ(EtOH) = 50% (v/v)
Neutralized, just before use, with KOH in presence of 0.3 mL phenolphthalein solution per 100 mL solvent mixture. |
| Phenolphthalein solution |
Phenolphthalein in ethanol, β(Phenolphtalein) = 1 g / 100 mL. |
Standard
| . |
. |
| Benzoic acid |
Benzoic acid is dried in a desiccator overnight. |
Sample Preparation
No sample preparation is needed.
Analysis
Titer
100 – 120 mg benzoic acid is weighed into the titration vessel and dissolved in 50 mL ethanol. Next the solution is titrated using c(KOH) = 0.1 mol/L until the first equivalence point has passed.
Blank
50 mL solvent mixture is added into a 150 mL beaker. After a 30 second pause, the solution is titrated until the first equivalence point using alcoholic c(KOH) = 0.1 mol/L.
Sample
See table below. An appropriate sample amount is weighed into a 150 mL beaker. 50 mL solvent mixture is then added and the sample dissolved. The solution is titrated until the first equivalence point using alcoholic c(KOH) = 0.1 mol/L after a pause of 30 seconds.
Amount of Sample Depending on the Expected Acid Value
| Expected AV / mg KOH / g |
Sample amount / g |
Accuracy / g |
| < 7 |
6 – 8 |
0.001 |
| 7 – 15 |
2.5 |
0.001 |
| 15 – 75 |
0.5 |
0.0001 |
| > 75 |
0.2 |
0.0001 |
Parameters
Titer
| . |
. |
| Mode |
DET U |
| Signal drift |
50 mV/min |
| Max. waiting time |
26 s |
| Meas. point density |
4 |
| Min. increment |
10 μL |
| Max. increment |
off |
| EP criterion |
5 |
| EP recognition |
all |
Sample
| . |
. |
| Mode |
DET U |
| Signal drift |
20 mV/min |
| Max. waiting time |
38 s |
| Meas. point density |
4 |
| Min. increment |
10 μL |
| Max. increment |
off |
| EP criterion |
5 |
| EP recognition |
all |
Calculation
Titer

Acid Value
Example Determination
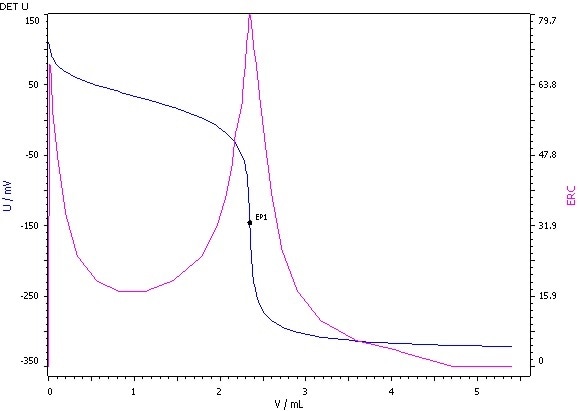
Figure 1: Determination of the acid value (blue = titration curve, pink = ERC).
Comments
- ASTM D7253 is similar to ASTM D4662. The key differences are:
- Utilization of c(KOH in methanol) = 0.02 mol/L
- Utilization of 100 mL isopropanol instead of solvent mixture
- Utilization of 50 – 60 g sample
- Decrease the sample size or employ a solvent mixture of one volume ethanol and three volumes tert-butyl methyl ether or toluene for materials which are less soluble. This mixture should also be neutralized.
References
- ASTM D4662 Standard Test Methods for Polyurethane Raw Materials. Determination of Acid and Alkalinity Numbers of Polyols.
- ASTM D7253 Standard Test Method for Polyurethane Raw Materials: Determination of Acidity as Acid Number for Polyether Polyols.
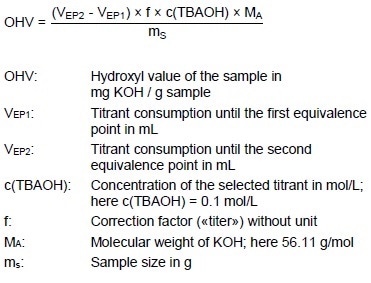
Hydroxyl Value (OHV)
Summary
The hydroxyl value supplies information about the degree of esterification within the sample and is given in mg KOH per g sample.
Instruments
- DIS-Cover
- Sample changer
- Magnetic stirrer for sample changer
- 2x Buret 20 mL (reaction solution, titrant)
- Titrator with DET mode
- 1x Buret 50 mL (acetonitrile)
- 1x Buret 2 mL (deionized water)
Electrodes
| . |
. |
| Solvotrode easyClean |
6.0229.010 |
Reagents
- Potassium hydrogen phthalate, KHP, pa.
- Acetonitrile, HPLC quality
- Toluene-4-sulfonyl-isocyanate (TSI), purum
Solutions
Titrant
Tetrabutyl ammonium hydroxide, c(TBAOH) = 0.1 mol/L in isopropanol/methanol, Φ(MeOH) = 50 % (v/v), this solution should be bought from a supplier if possible.
TSI solution
250 mL acetonitrile approximately is put into a 500 mL volumetric flask and 20 mL TSI is added. Next, the flask is filled up to the mark with acetonitrile and mixed. The solution vigorously reacts with water, therefore it is recommended to work under protective gas in a fume hood. The reaction solution is stable for around one month.
Standard
KHP
KHP is dried in a drying oven at 120 °C for two hours and left to cool down in a desiccator for at least an hour.
Sample Preparation
No sample preparation needed.
Analysis
Titer
60 mL deionized water is added to around 180 mg KHP. The suspension is then stirred for approximately one minute to dissolve the KHP. Next, the solution is titrated until the first equivalence point using c(TBAOH) = 0.1 mol/L.
Sample
An appropriate amount of sample (see the below calculation) is weighed into the titration vessel and dissolved in 10 mL acetonitrile, the solution is then stirred for 30 seconds using stirring rate 8. The sample is covered after 10.0 mL TSI solution are added, the mixture is then stirred using stirring rate 4.
0.5 mL deionized water is added after 5 minutes and the lid is closed again before the solution is stirred for another 60 seconds at stirring rate 4. 40 mL acetonitrile is added and the solution is titrated until after the second end point with c(TBAOH) = 0.1 mol/L. The vessel and buret are rinsed first with acetonitrile, then with deionized water after each titration. The electrode is conditioned for 1 minute in deionized water.

Parameters
Titer
| . |
. |
| Mode |
DET U |
| Pause |
30 s |
| Signal drift |
50 mV/min |
| Max. waiting time |
26 s |
| Meas. point density |
4 |
| Min. increment |
10 μL |
| Max. increment |
off |
| EP criterion |
5 |
| EP recognition |
greatest |
Sample
| . |
. |
| Mode |
DET U |
| Pause |
30 s |
| Signal drift |
50 mV/min |
| Max. waiting time |
26 s |
| Meas. point density |
4 |
| Min. increment |
10 μL |
| Max. increment |
off |
| EP criterion |
5 |
| EP recognition |
all |
Calculation
Titer

Sample
Example Determination
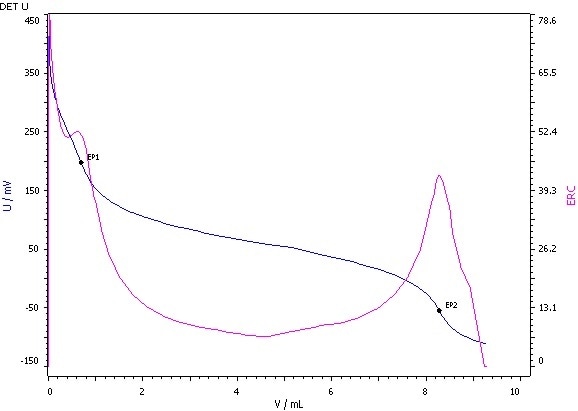
Figure 2: Determination of the hydroxyl value (blue = titration curve, pink = ERC).
Comments
- Use a 15 to 20 g sample for samples with an expected hydroxyl value of 2 or lower.
- In order to ensure the complete immersion of the electrode, 40 mL instead of 30 mL acetonitrile are added after the preparation.
- As alcohols react with the TSI, too high results are obtained when utilizing e.g., ethanol for cleaning. So, acetonitrile is recommended as solvent, acetone is another possible solvent.
- The ASTM technique is shown here, as it is quicker (12 minutes) than the DIN method (40 minutes).
- Do not dab the tip of an electrode with a tissue, as this damages the electrode.
References
- ASTM E1899 Standard test method for hydroxyl groups using reaction with p-toluene sulfonyl isocyante (TSI) and potentiometric titration with tetrabutyl ammonium hydroxide
- DIN 53240-3 Binders for paints and varnishes - Determination of hydroxyl value - Part 3: Rapid test
Isocyanates Content (NCO)
Summary
Diisocyantes are utilized for manufacturing polyurethanes. As the value shows the concentration of the active NCO groups in the sample, the isocyanate (NCO) content is a crucial quality parameter. The NCO content is stated in g of isocyanate per 100 g of sample. In this instance, the calculation according to EN ISO 14896 is shown, as less chemicals are used in comparison to ASTM D5155 and ASTM D2572, making it the more environmentally friendly technique.
Instruments
- 2x Buret 50 mL (toluene, acetone)
- 2x Buret 20 mL (reaction solution, titrant)
- DIS-Cover
- Sample changer
- Magnetic stirrer for sample changer
- Titrator with DET mode
Electrodes
| . |
. |
| Solvotrode easyClean |
6.0229.010 |
Reagents
- Dibutylamine
- Toluene (dried over molecular sieve)
- Acetone
Solutions
Titrant
c(HCl) = 1 mol/L aqueous. This solution should be bought from a supplier if possible.
Reaction Solution
c(dibutylamine) = 1 mol/L in toluene (dried over molecular sieve)
Standard
TRIS
TRIS is dried at 105 °C overnight in a drying oven and left to cool down in a desiccator for minimum of one hour.
Sample Preparation
No sample preparation required.
Analysis
Titer
Approximately 420 mg TRIS is weighed into a titration vessel. 20 mL deionized water and 50 mL acetone are added. After a 20-second pause, the solution is titrated with c(HCl) = 1.0 mol/L until the first equivalence point. The electrode membrane is rehydrated for one minute in deionized water in between measurements.
Blank
A blank sample is treated and titrated in the exact same way as the actual sample without sample.
Sample
Weigh out an appropriate amount of the sample (around 2 g) and then add 30 mL toluene to dissolve it. Add 18.0 mL reaction solution, cover the vessel, and react for 10 minutes on the magnetic stirrer. Next, 30 mL of acetone should be added and the excess of dibutylamine is back titrated with c(HCl) = 1 mol/L.
Parameters
Titer
| . |
. |
| Mode |
DET U |
| Pause |
20 s |
| Signal drift |
20 mV/min |
| Max. waiting time |
38 s |
| Meas. point density |
4 |
| Min. increment |
50 μL |
| Max. increment |
off |
| EP criterion |
5 |
| EP recognition |
greatest |
Blank
| . |
. |
| Mode |
DET U |
| Signal drift |
20 mV/min |
| Max. waiting time |
38 s |
| Meas. point density |
4 |
| Min. increment |
10 μL |
| Max. increment |
off |
| EP criterion |
5 |
| EP recognition |
all |
Sample
| . |
. |
| Mode |
DET U |
| Signal drift |
20 mV/min |
| Max. waiting time |
38 s |
| Meas. point density |
4 |
| Min. increment |
10 μL |
| Max. increment |
off |
| EP criterion |
5 |
| EP recognition |
all |
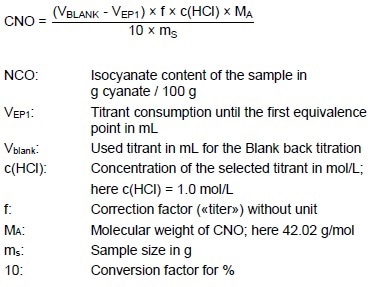
Calculation
Titer

Sample
Example Determination
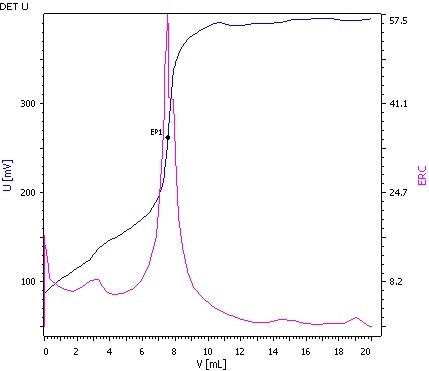
Figure 3: Determination of the Isocyanate value (blue = titration curve, pink = ERC).
Comments
- Turbidity will be experienced in the titrations. Inhomogeneity can be tolerated without adversely affecting the results if the mixtures are agitated vigorously. Otherwise, methanolic HCl could be employed as titrant.
- Special precautions in sampling must be taken since organic isocyanates react with atmospheric moisture. Even when performed quickly, usual sampling techniques can cause contamination of the sample with insoluble ureas; so, cover the sample with a dry inert gas (e.g., argon, nitrogen, or dried air) at all times.
- Warning – when absorbed through the skin, or when the vapors are breathed in, organic isocyanates are hazardous. Adequate ventilation must be provided and protective gloves and eyeglasses worn when needed.
- ASTM D2572 is similar to EN ISO 14896. The differences are:
- Concentration of titrant c(HCl) = 01 mol/L
- Use of isopropanol (100 mL) instead of acetone
- Reaction time of 15 minutes
- Concentration of reaction solution c(dibutylamine) = 01 mol/L
- Different amounts of toluene (25 mL) and reaction solution (25 mL) and smaller sample size (0.1 g)
- ASTM D5155 is similar to EN ISO 14896. The differences are:
- Use of isopropanol (225 mL) instead of acetone
- Reaction time of 15 minutes
- Concentration of reaction solution c(dibutylamine) = 2 mol/L
- Different amounts of toluene (50 mL) and reaction solution (50 mL) and higher sample size (6.5 - 7 g)
References
- EN ISO 14896 Plastics — Polyurethane raw materials - Determination of isocyanate content
- ASTM D5155 Standard Test Methods for Polyurethane Raw Materials: Determination of the Isocyanate Content of Aromatic Isocyanates
- ASTM D2572 Standard Test Method for Isocyanate Groups in Urethane Materials or Prepolymers
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.