In determination of the capacity of water to neutralize acidic pollution, measurement of alkalinity is of paramount importance. Because of human activities, the alkalinity of rivers and streams may be affected in the long-term.
Estimation of the alkalinity in brines, seawater, and brackish water using ASTM D3875 compliant automatic potentiometric titration method is outlined in this application note. The method uses hydrochloride acid as the titrant.
Sample
Seawater, brackish water and brine
Sample Preparation
No sample preparation is required.
Analysis
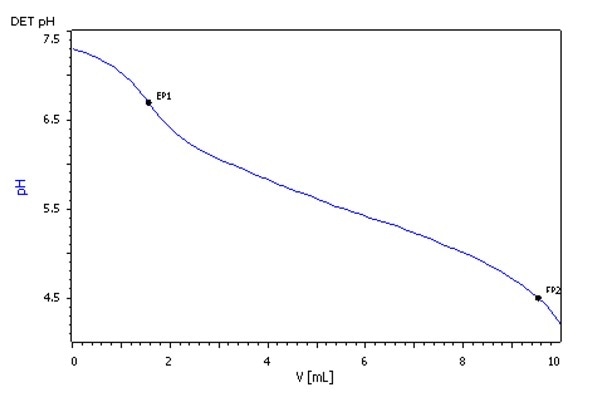
Approximately 80 mL of brine, seawater, or brackish water sample is taken in a beaker using a pipette. Employing the Unitrode, titration is then carried out using HCl with 0.1 mol/L concentration to achieve a pH-value of 3.0. Two endpoints, one at pH 8.1 and another at pH 4.5 are determined and used for the estimation.
Configuration
| . |
. |
| 905 Titrando |
2.905.0010 |
| 800 Dosino, 2x |
2.800.0010 |
| 814 USB Sample Processor (1T/1P) |
2.814.0010 |
| 772 Pump Unit |
2.772.0110 |
| Dosing unit 50 mL, (sample) |
6.3032.250 |
| Dosing unit 10 mL, (titrant) |
6.3032.210 |
| 802 Rod stirrer |
2.802.0020 |
| Stirring propeller |
6.1909.050 |
| Sample rack, 16 x 150 mL |
6.2041.320 |
| Titration head, 6x NS 14 and 3x NS 9 openings |
6.1458.010 |
| Sample beakers, glass, 16 x 150 mL |
- |
| Unitrode with Pt 1000 |
6.0258.600 |
| Electrode cable for plug in head U/plug F, 2x2 mm B, 1m |
6.2104.600 |
| tiamoTM 2.5 |
6.6056.252 |
Solutions
| . |
. |
| Titrant |
Hydrochloride acid, c(HCl) = 0.1 mol/L |
Parameters
| . |
. |
| Mode |
DET U |
| Stirring rate |
10 |
| Signal drift |
50 mV/min |
| Min. waiting time |
0 seconds |
| Max. waiting time |
26 seconds |
| Meas. point density |
4 |
| Dosing rate |
Maximum |
| Min. increment |
10 μL |
| Max. increment |
Off |
| EP criterion |
5 |
| EP recognition |
All |
| Stop volume |
5 mL |
| Stop pH |
3.0 |
| Fixed endpoint 1 |
pH = 8.1 |
| Fixed endpoint 2 |
pH = 4.5 |
Results
| Sample |
Alkalinity |
| HCO3- / mg/L |
s(rel) / % |
CO32- / mg/L |
s(rel) / % |
| Seawater |
136 |
0.2 |
2.4 |
5.7 |
| Brackish water |
8.5 |
0.8 |
0 |
- |
| Brine |
743 |
0.1 |
0 |
- |
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.