All antibodies display a feature on their variable portion that is a unique set of antigenic determinants (epitopes), known as the idiotope. An antibody that is anti-idiotypic (Anti-ID) will bind to the idiotope of another antibody, usually an antibody drug, which makes it an incredibly powerful tool in the development of antibody drugs, especially for immunogenicity and PK/PD analysis.
ACROBiosystems has recently developed a series of high-affinity anti-idiotypic antibodies to assist in preclinical/clinical immunogenicity and PK analysis. Their pipeline covers five hot targets including adalimumab, rituximab, cetuximab, trastuzumab, and bevacizumab. They also provide assay protocols which can be applied to different application scenarios, to help along the drug development process.
Product List
| Cat.No. |
Antigen |
Neutralizing Activity |
Affinity KD, nM |
Application |
| TRB-Y1 |
Trastuzumab F(ab')2 |
Neutralizing Antibody |
0.296 |
PK bridging ELISA with TRB-BY5
Neutralizing assay
Indirect ELISA |
| TRB-Y7 |
Trastuzumab F(ab')2 |
Neutralizing Antibody |
0.452 |
ADA assay
Neutralizing assay
Indirect ELISA |
| ADB-Y19 |
Adalimumab F(ab’)2 |
Neutralizing Antibody |
0.0013 |
ADA assay
Neutralizing assay
Indirect ELISA |
| CEB-Y27 |
Cetuximab F(ab’)2 |
Neutralizing Antibody |
0.007 |
ADA assay
Neutralizing assay
Indirect ELISA |
| CEB-Y28 |
Cetuximab F(ab’)2 |
Neutralizing Antibody |
0.0015 |
ADA assay
Neutralizing assay
Indirect ELISA |
| CEB-Y31 |
Cetuximab F(ab')2 |
Non-Neutralizing Antibody |
0.421 |
ADA assay
Indirect ELISA |
| RIB-Y35 |
Rituximab F(ab’)2 |
Neutralizing Antibody |
0.03 |
ADA assay
Neutralizing assay
Indirect ELISA |
| RIB-Y36 |
Rituximab F(ab')2 |
Neutralizing Antibody |
0.01 |
ADA assay
Neutralizing assay
Indirect ELISA |
| RIB-Y37 |
Rituximab F(ab')2 |
Neutralizing Antibody |
/ |
PK bridging ELISA with RIB-BY35
Neutralizing assay
Indirect ELISA |
| BEB-Y12 |
Bevacizumab F(ab’)2 |
Neutralizing Antibody |
0.0828 |
Neutralizing assay
Indirect ELISA |
| BEB-Y9 |
Bevacizumab F(ab’)2 |
Neutralizing Antibody |
1.92 |
ADA assay
Indirect ELISA |
ADA Assay - Perfect as CalibratorApplication
Therapeutic proteins such as monoclonal antibodies are currently vital for treating cancer, autoimmune disease, and other diseases. The structure of protein contains potential B-cell and T-cell epitopes, making it intrinsically immunogenic. Therefore, therapeutic proteins have the potential to induce Anti-Drug Antibodies (ADA) even if the protein has the same amino acid sequence as endogenous human proteins.
If ADA starts to arise in patients it can potentially lead to loss of efficacy and/or adverse events. This means that throughout the development of therapeutic protein products, researchers must carry out immunogenicity risk assessment and risk-mitigating strategies.
At the moment it is extremely time-consuming to develop a mono/multi-clonal antibody in-house as a positive control for ADA assay. To solve this problem, ACROBiosystems developed a series of anti-drug antibody standards for ADA assays.
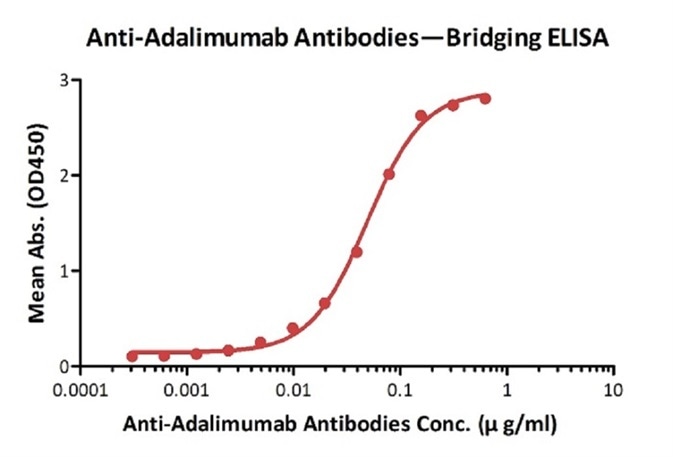
Anti-Rituximab Antibodies bridging ELISA for Anti-Drug Antibody (ADA) assay development. Immobilized rituximab at 5 µg/ml, add increasing concentrations of Anti-Rituximab Antibodies (Cat. No. RIB-Y35, 10% human serum) and then add biotinylated rituximab at 5 µg/ml. Detection was performed using HRP-conjugated streptavidin with a sensitivity of 20 ng/mL. Image credit: ACRO Biosystems
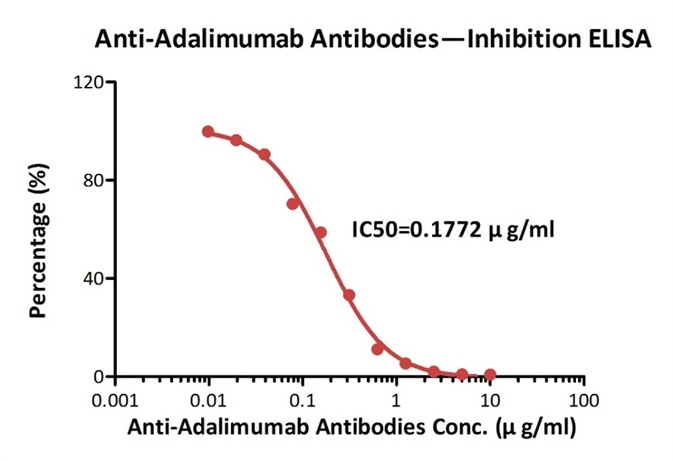
Measured by its neutralizing ability in FACS. The data shows that the binding of rituximab to 293F overexpressing CD20 was inhibited by increasing concentrations of Anti-Rituximab Antibodies (Cat. No. RIB-Y35). The concentration of rituximab used is 10 ng/ml. The IC50 is 0.013 μg/ml. Image credit: ACRO Biosystems
PK Assay – Quantitative Analysis of Therapeutic Antibody in Matrix
An enzyme-linked immunosorbent assay, or ELISA, is the most commonly used technology for quantitative analysis of therapeutic antibody in matrix. There are several ways to carry such an assay out including: indirect ELISA, competitive ELISA, sandwich ELISA or bridging ELISA. Out of this set, bridging ELISA is usually the preferred option, due to its low non-specific background and high sensitivity. As seen from the data, using goat anti-human IgG as secondary antibody leads to high background due to unspecific binding, and therefore makes it necessary to perform pre-dilution before analyses.
On the other hand, the bridging ELISA by anti-idiotypic antibodies minimizes the background interference by using HRP-conjugated Streptavidin for secondary detection. ACROBiosystems has developed a series of anti-idiotypic antibodies that are used for bridging ELISA.
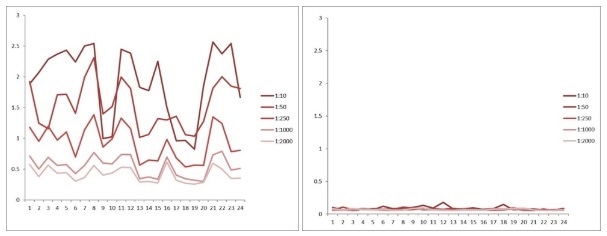
Comparison between anti-idiotypic capture ELISA and anti-idiotypic bridging ELISA for rituximab detection in patient samples. Left: anti-idiotypic capture ELISA; Right: anti-idiotypic bridging ELISA. Image credit: ACRO Biosystems
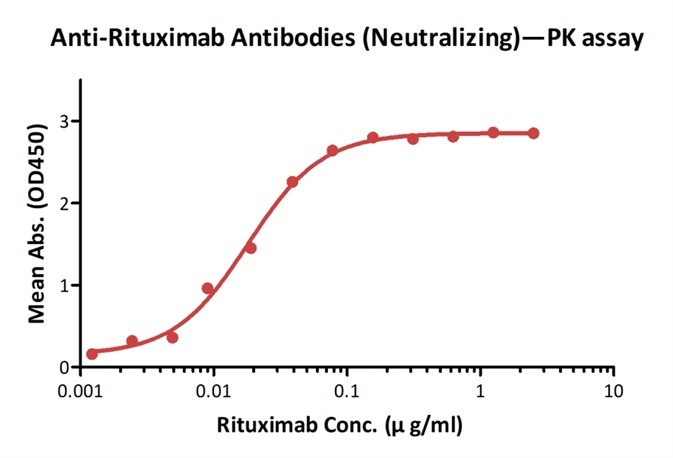
Detection of rituximab by bridging ELISA in serum. Immobilized Anti-Rituximab Antibodies (Cat. No. RIB-Y37) at 2 μg/ml, added increasing concentrations of Rituximab (10% human serum) and then added biotinylated Anti-Rituximab Antibodies (Cat. No. RIB-BY35) at 1 μg/ml. Detection was performed using HRP-conjugated streptavidin with a sensitivity of 1 ng/ml. Image credit: ACRO Biosystems
High Affinity
The measure of the strength of interaction between an antigen and an antibody is known as the affinity, which can be described as KD. The smaller KD represents a stronger affinity. ACROBiosystems measured the affinity of all the anti-idiotype antibodies using the SPR method. This piece of data helps to predict the sensitivity in assay development.
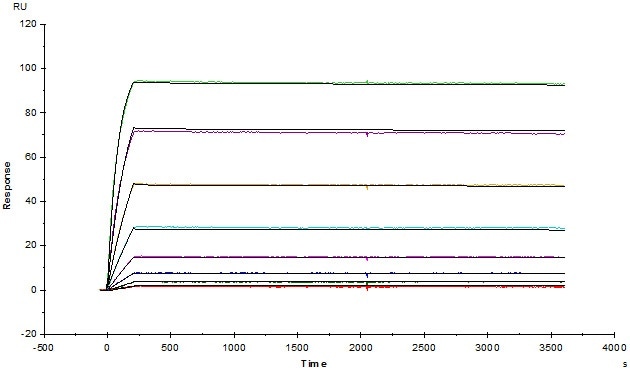
Anti-Adalimumab Antibodies (mouse IgG1, Cat. No. ADB-Y19) captured on CM5 chip via anti-mouse antibodies surface, can bind human adalimumab with an affinity constant of 1.36 pM. Image credit: ACRO Biosystems
High Specificity
As well as the affinity, AcroBiosystems ensures the specificity of each anti-idiotype antibody product before it goes on the market.
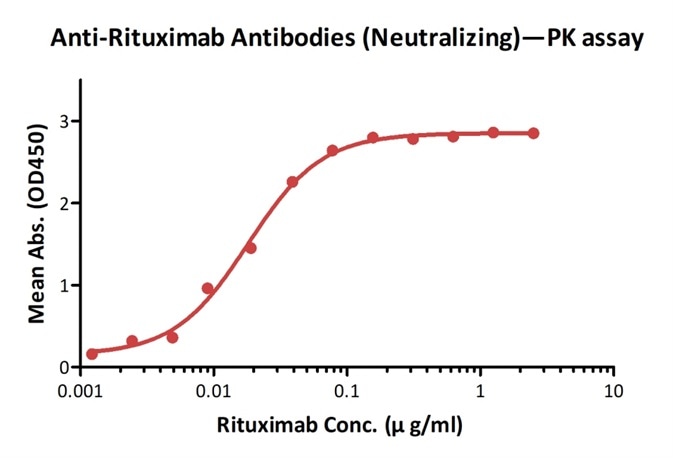
Demonstration of the specificity of Anti-Cetuximab Antibodies (Cat. No. CEB-Y28) to the cetuximab. Image credit: ACRO Biosystems
High Stability
To ensure the stabilities of their products before they go on the market, ACROBiosystems performs accelerated stability tests and freeze-thaw tests.
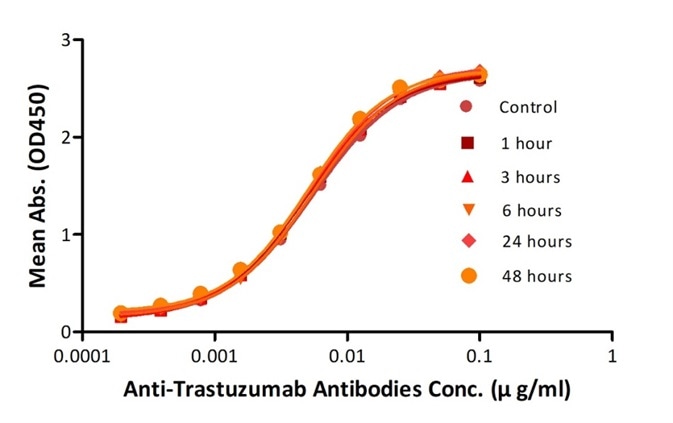
Reconstituted Anti-Trastuzumab Antibodies were diluted to 0.4 mg/ml, aliquoted and placed at 37°C. Aliquots were removed from 37°C at every time point and placed at 4°C along with the control. No significant loss of activity was observed. Image credit: ACRO Biosystems
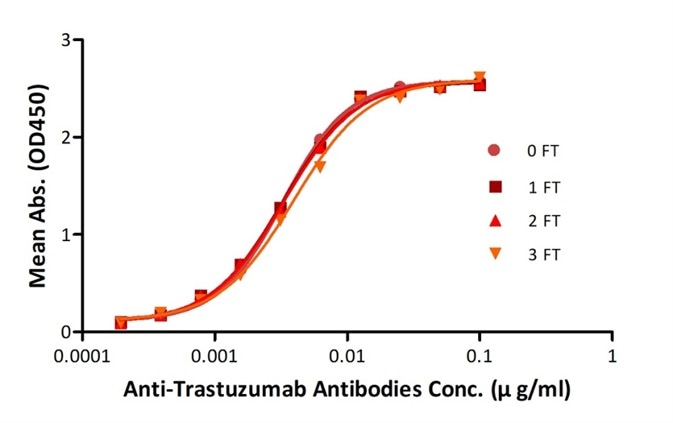
Anti-Trastuzumab Antibodies were subjected to the indicated number of freeze-thaw cycles (FT). No significant loss of activity was observed. Image credit: ACRO Biosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.