Immune checkpoint (CKP) drugs are leading the way in current immunotherapies, having gained popularity in immune therapy due to their exceptional clinical efficacy capabilities. The considerable anti-cancer potential of CPK drugs has meant that it has established itself at the forefront of pharmaceutical pipelines across the globe.
In this highly competitive field, a detailed evaluation of promising candidates is required to improve the chance of gaining FDA drug approval. Throughout the research and development phase, therapeutic efficacy is the principal indicator for candidate drug selection.
However, directly performing in vivo studies could prove therapeutic efficacy and correlate better with ongoing clinical studies. This leads to questions about why these studies are required and should be conducted.
A primary purpose for in vitro studies is their capability to offer early data support, specifically when formed in conjunction with primary cells. Although this might initially appear redundant to in vivo studies, there is a key distinction. In vitro studies have experimentally controlled conditions, which offer indisputable evidence supporting the mechanism of action (MoA) and therapeutic efficacy.
For CKP drugs, these assays focus on several MoA evaluations: T-cell activation and expansion, cell-mediated activation, antibody-dependent cell-mediated cytotoxicity (ADCC), and more. This article explores the three main in vitro assays shedding light on MoAs and making reference to specific cases.
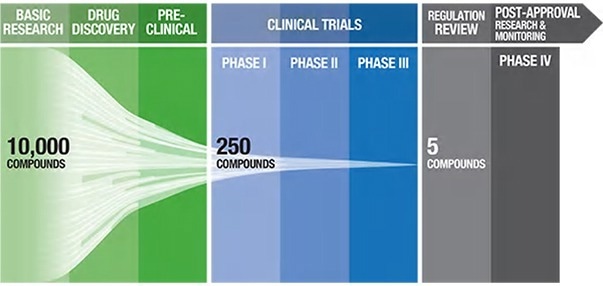
Figure 1. Pharmaceutical pipeline highlighting the number of potential candidate drugs to regulatory approval1. Image Credit: ACROBiosystems
Mechanism of action 1: T-cell activation and proliferation
T-cell activation and proliferation are key MoA in CKP agonist drugs that are dependent on the promotion of adaptive immune response to coordinate an overall immune response against cancer cells. For instance, Kuang et al. conducted numerous in vitro assays on their anti-OX40 antibody (IBI101) that targets colorectal adenocarcinoma.1
T-cell activation was determined through the quantitation of cytokine secretion. This involved the preparation of a 96-well plate coated with both anti-CD3 and IBI101, with CD4+ T-cells introduced into each well in addition to cell media and anti-CD28 antibody.
After being incubated for three days, IL-2 secretion was then quantified using ELISA. For T-cell proliferation, Far Red dilution was employed for determination. PMBCs dyed using Far Red fluorescent reactive dye were transferred into anti-CD3 coated well plates which contained anti-CD28.
Cells were then incubated for four days with IBI101 and human IgG antibodies and stained with anti-human CD4/CD8 before conducting analysis using flow cytometry.
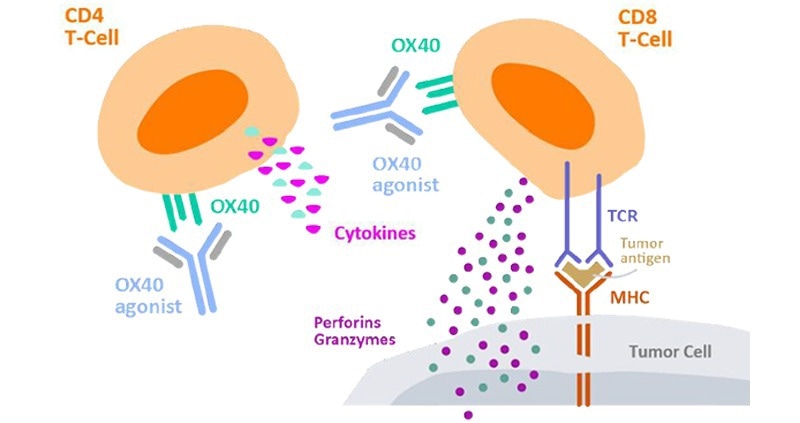
Figure 2. Cytokine secretion by CD4+ T cells to measure cell activation2. Image Credit: ACROBiosystems
Mechanism of action 2: Cell-mediated T-cell activation
CKP inhibitors, as opposed to agonists, work by blocking a pathway that arbitrates T-cell function, necessitating a more indirect testing method for mixed lymphocyte reaction (MLR). This assay was carried out by Wang et al. to determine Nivolumab, an anti-PD-1 antibody that functions as a CKP inhibitor.5 To perform this assay, dendritic cells (DCs) were co-cultured with T-cells. PD-L1 is expressed in abundance on the surface of DC cells which bonds with PD-1 on T-cells to modulate T-cell activation.
The introduction of Nivolumab in combination with signaling cytokines such as IL-4 and GM-CSF shows an expansion of T-cells despite the presence of immune-modulating DCs. The consequent T-cell expansion is evaluated through cytokine secretion (IFN-γ) using a cytokine ELISA kit.
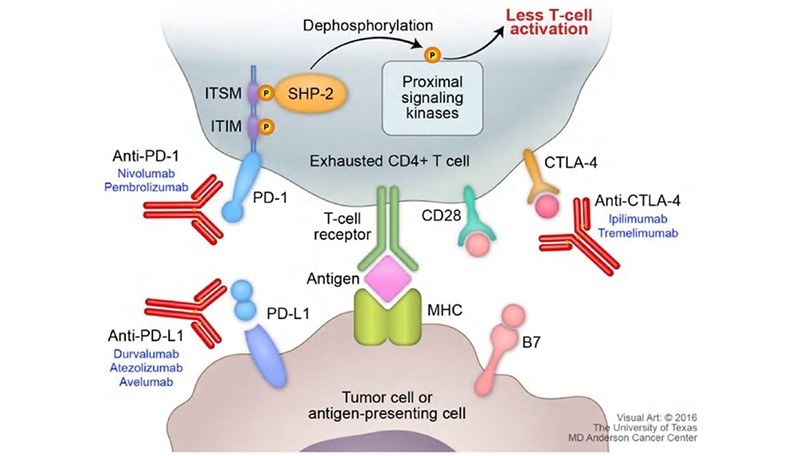
Figure 3. Figure on PD-1 pathway with cytokine secretion4. Image Credit: ACROBiosystems
Mechanism of action 3: Antibody-dependent cell-mediated cytotoxicity
The third and final MoA under discussion is antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC is a natural immune mechanism where a particular antibody binds to a target cell while engaging with NK cells via FCγR to initiate cell lysis.
The application of antibody drugs allows this mechanism to be used to target specific cells. One such instance is ATOR-1015, a bispecific antibody presented in the work of Kvarnhammar et al.6
ATOR-1015 is responsible for targeting CTLA-4 and OX40, which are upregulated on activated T-cells, specifically regulatory T-cells (Tregs), that reside in the tumor microenvironment. With the application of antibody drugs, this mechanism can be used for targeting specific cells.
One suitable example is ATOR-1015, a bispecific antibody introduced by Kvarnhammar et al.6 Residing in the tumor microenvironment CTLA-4 and OX40 are targeted by ATOR-1015. CTLA-4 and OX40 are upregulated on activated T-cells, particularly regulatory T-cells (Tregs). Treg depletion takes place through ADCC with ATOR-1015 binding to FCγRIIIa.
To assess this MoA, an in vitro assay employing isolated NK cells with Tregs was conducted. Tregs were initially activated via anti-CD3/CD28 magnetic beads for the expression of CTLA-4 and OX40. Subsequently, NK cells are cultured using the activated Tregs and ATOR-1015.
As a result, ATOR-1015 bonds to both NK cells and Tregs, inducing cell lysis of OX-40 and CTLA-4 expressing Tregs measured via lactate dehydrogenase content.
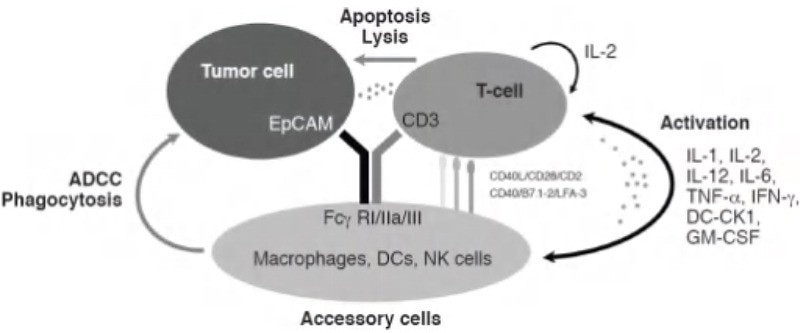
Figure 4. ADCC mechanism of action triggered by bispecific antibodies.5 Image Credit: ACROBiosystems
Discussion
In vitro functional assays are crucial for the practical evaluation of a candidate CKP antibody drug in the initial stages of research and development. These assays offer scientific evidence for validating antibody activity, understanding MoAs, and providing preliminary evidence that supports therapeutic efficacy. As such, they play a key role in the decision-making process in drug candidate selection.
Although in vitro assays can vary significantly between each type of drug, ACROBiosystems’ broad catalog of suitable products helps in the development of in vitro assays, including cytokines, quantitative cytokine ELISA kits, immune checkpoint recombinant proteins, anti-CD3 antibodies, anti-CD28 antibodies, anti-CD3/CD28 antibody-coupled magnetic beads, and many more.
References
- WEHI. Drug Discovery. 2022. [Online] Available at: https://www.wehi.edu.au/research/research-technologies/drug-discovery
- Abbvie Oncology. 2021 [Online] Available at: https://www.abbviescience.com/.
- Kuang Z., Liu J., et al. Development and Characterization of a novel anti-OX40 antibody for potent immune activation. Cancer Immunol Immunother. 2020, 69(6):939-950.
- Schvartsman G., Massarelli E., et al. Checkpoint inhibitors in lung cancer: Latest developments and clinical potential. Ther. Adv. in Med. Oncol. 8(6).
- Wang C., Korman A., et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014. 2(9), 846-856.
- Kvarnhammar A., Ellmark P., et al. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J Immunotherapy of Cancer. 2019, 7:103.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.