Immunology is a rich and diverse field involving numerous different elements and perspectives. For the majority of cases, research has heavily emphasized the details: discovering and elucidating signaling pathways that stimulate, suppress, or regulate immune cell function.
In other cases, immunology focuses on a cellular-level perspective: immune cell interactions and responses.
The true end-goal of all of each of these research perspectives, however, is the same: the identification of novel therapeutic strategies to overcome an erratic immune system or new targets for immunotherapy: otherwise considered a vital aspect of translational medicine.
As immunology continues to uncover information collected from molecular and cellular perspectives, scaling up this information to the tissue/organ level necessitates a more complicated modeling system that can be hard to reproduce.
There are still many challenges at this stage, particularly when it comes to forming the optimal conditions to drive stem cell growth without differentiation and understanding how cells assemble into the correct structure.
Basement membranes bridge the gap between each perspective: a complex composition of extracellular matrix proteins that drive cellular growth and differentiation by providing cells with both structural and chemical signals. This membrane is ever-present – acting as a bridge or surrounding material in order to provide cellular structure to cells.
Basement membrane extracts: What are they?
Basement membrane extracts can be a synthetic or natural, randomly interwoven composition of extracellular matrix proteins layered in intricate sheets to provide a three-dimensional structure. Gene-edited mouse tumor cells, usually mouse sarcoma tumors, generate natural basement membrane extracts rich in extracellular matrix proteins.
Further purification steps can also be taken to produce a ‘reduced growth factor’ extract, which minimizes the chemical signals present in the matrix. The resulting structure forms a hydrogel composed of laminin, fibronectin, and various other proteins, which can be used in cell proliferation, cell differentiation, or even to direct cell health, behavior, or growth dynamics.
However, it is essential to note that basement membrane extracts are prone to lot-to-lot diversity and can be difficult to work with. Tailoring the right concentration and protocol for any application, alongside product quality and consistency, is, therefore, critical when it comes to developing a successful model.
Cell culturing in 2 or 3 dimensions
Developing the right hydrogel using basement membrane extracts is challenging yet crucial for successful culturing. Cell behavior can differ significantly depending on the concentration or the integrity of the hydrogel formed. A thin layer coating works best for 2D cell culture, whether for iPSCs or other primary cells.
Maintaining stem cell stemness and promoting surface attachment is critical, which is why a reduced growth factor extract should be utilized to minimize differentiation influences from growth factors or intrinsic cytokines.
However, the addition of other stem cell growth factors may be required for stem cell differentiation. Differentiation can be induced towards the targeted cell type by mixing growth factors within the gel before plating stem cells.
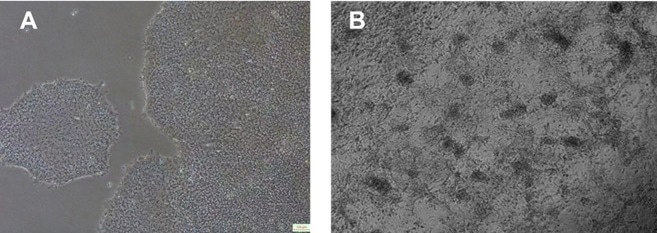
Figure 1. Differentiation and expansion of (a) induced pluripotent stem cells (iPSCs) into (b) myocardial cell precursors after induction. Image Credit: ACROBiosystems
On the other hand, 3D cell culturing maximizes the gel-like properties of basement gel extracts, providing the necessary cellular structure to allow organoids, or organ precursors, to form.
Rather than being derived from a cell line, organoids are made up of various types of cells that correspond to their starting material composition, providing an in vitro ‘snapshot’ of cell diversity and structure. This information is invaluable in immunology disease modeling, particularly when it comes to looking deeper into cell tissue or at more macro-level interactions.
When culturing organoids, tissue fragments are mostly used as a starting material. Depending on the desired organoid type, thin-layer, and thick-layer gels can provide the optimum environment for 3D cellular growth, including mouse intestinal organoids, lung tracheal organoids, and hepatobiliary organoids.
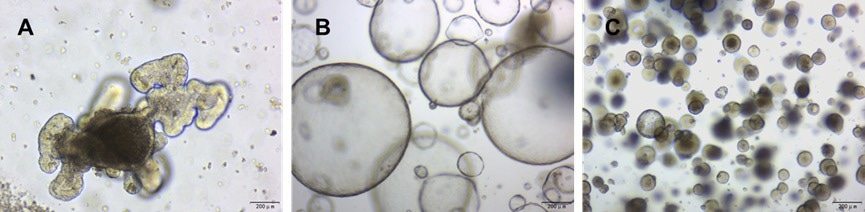
Figure 2. Culturing of (a) mouse intestinal organoids, (b) mouse liver ductal organoids, and (c) mouse airway organoids in a 70% Matrigengel Matrix GFR gel. Image Credit: ACROBiosystems
Certain organoids can be more difficult to culture; human tumor organoids fall into this category. However, organoid structure can be improved by mixing the starting material with the basement membrane extract before plating and gelling.
A denser extracellular matrix layer and thicker gel layer provide a better scaffolding for cell attachment and growth. Similarly, organoid growth factors can be added to help further promote growth and accurate cell diversity.
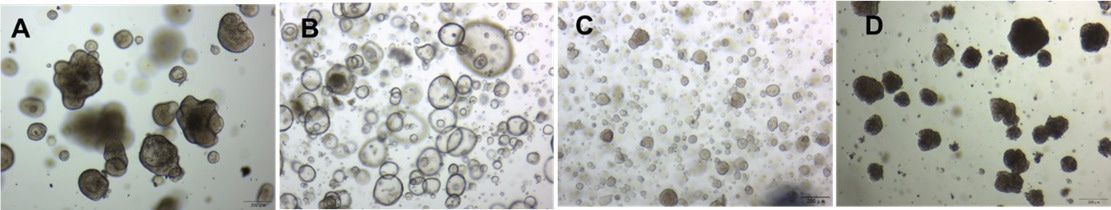
Figure 3. Culturing of (a) colorectal cancer organoids, (b) gastric cancer organoids, and (c) breast cancer organoids, and (d)hepatocellular carcinoma organoids in a 70% Matrigengel Matrix Organoid gel. Image Credit: ACROBiosystems
Disease modeling – angiogenesis and xenografting
Although organoids are a good cellular-level precursor model for immunology research, basement membrane extracts are also critical in macro-scale disease modeling, such as angiogenesis and patient-derived tumor xenografting.
Certain modeling studies, such as angiogenesis, mimic the physiological extracellular matrix that can promote structure formation; in the case of angiogenesis, this is blood vessel formation.
This is an especially critical model for tumor formation to make the connection between how structure formation (tissue level) can be influenced by cell signals (molecular level) and identifying key targets to help overcome this effect.
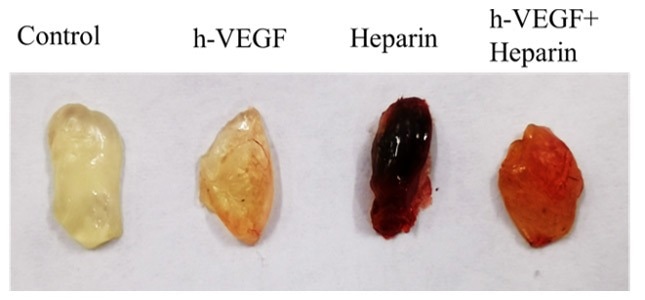
Figure 4. Subcutaneous injection of Matrigengel plug to assess the angiogenic activity of different compounds mixed with VEGF, Heparin, or both; both of which are known angiogenesis-inducing factors. The intensity of red denotes the degree of blood vessel formation. Image Credit: ACROBiosystems
On the other hand, patient-derived xenografting (otherwise known as PDX) is one of the key precursors for personalized medicine, which operates through patient-specific diagnostics. Each tumor has its own specific markers and cell composition, much like how each person has their own individual features, which can make the tumor hard to treat.
A sample of the solid tumor is taken by PDX, which then recreates the tumor in a mouse model. In treatment planning, where mice can be subjected to various treatments, this is especially critical to identify the optimal treatment path and minimize refractory responses or relapse.
The basement membrane is a key component of PDX, which provides both growth factors and structure essential for tumor development.
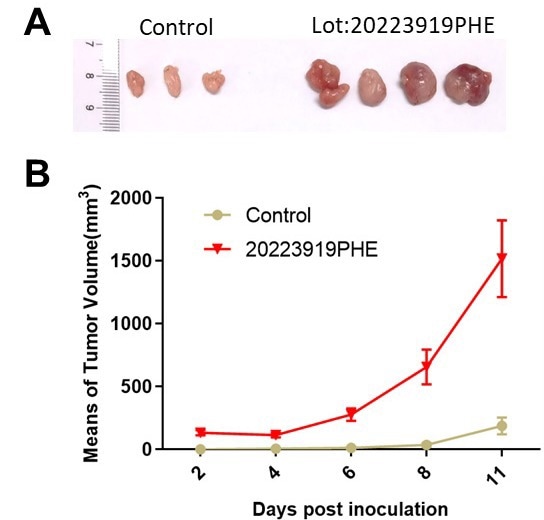
Figure 5. (a) Control vs. Matrigengel-assisted tumor grown in vivo using a mouse model. (b) Tumor volume growth after tumor cell inoculation between control (only tumor cells) and Matrigengel assisted growth. Image Credit: ACROBiosystems
Preparing the optimal basement membrane coating
The versatility of basement membrane results in a near-universal reagent that can be applied in a number of different applications, including organoid culturing, cell culturing, and, most importantly, disease modeling. However, it is vital that the right method is chosen and used.
Hydrogels with different properties, such as permeability, tensile strength, and adhesivity, are produced by various coating protocols. This can significantly affect their performance regarding the desired application.
This is especially the case with cells that are harder to culture and have more specific requirements for cell growth. Table 1 provides a rapid overview of the type of coating and desired application.
Table 1. Coating methodologies and their applications. Source: ACROBiosystems
| Coating Type |
Description |
Application |
| Thin Layer Coating |
A thin layer of Matrigengel Matrix is formed at the bottom of a well plate. |
|
| Thick Layer Coating |
A thick layer of Matrigengel Matrix is formed at the bottom of a well plate. |
|
| Sandwich Coating |
Two thick layers of Matrigengel Matrix are formed to surround the cultured cells. |
|
| Embedded Dome Coating |
Cells are mixed with liquid-form Matrigengel Matrix and plated in a dome-formation. |
|
Conclusion
In conclusion, basement membrane extracts represent a versatile reagent critical to both translational and basic research. Basement membranes are a vital component of understanding and interpreting the interplay between cells, molecules, and tissue layers, with effects ranging from cell culturing to disease modeling, particularly in disease pathology and immunology.
A vital thing to understand is that the majority of basement membrane extracts derive from gene-edited mouse tumor cells.
In comparison to synthetic extracts, using a more natural basement membrane extract generally provides better results.
However, basement membrane extracts are prone to batch-to-batch diversity in comparison to synthetic derivatives and can be harder to work with. It is, therefore, critical to tailor the right concentration and protocol for any application as well as focus on quality and consistency when trying to develop a successful model.
ACROBiosystems has recently partnered with Mogengel Bio through Acro Certify to offer consumers an application-validated basement membrane extract named Matrigengel Matrix.
For every batch manufactured, application studies are performed, such as organoid culture, stem cell culture, angiogenesis, tumor invasion, and tumor xenografts, to ensure that each product is capable of supporting the optimal application requirements.
References and further reading
- Arnaoutova, I., George, J., Kleinman, H. K. & Benton, G. Basement Membrane Matrix (BME) has Multiple Uses with Stem Cells. Stem Cell Rev. Reports 8, 163–169 (2012).
- Benton, G., Kleinman, H. K., George, J. & Arnaoutova, I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int. J. Cancer 128, 1751–1757 (2011).
- Aisenbrey, E. A. & Murphy, W. L. Synthetic alternatives to Matrigel. Nature Reviews Materials vol. 5 (2020).
- Youn, J. & Kim, D. S. Engineering porous membranes mimicking in vivo basement membrane for organ-on-chips applications. Biomicrofluidics 16, 1–7 (2022).
- Lopera Higuita, M., Shortreed, N. A., Dasari, S. & Griffiths, L. G. Basement Membrane of Tissue Engineered Extracellular Matrix Scaffolds Modulates Rapid Human Endothelial Cell Recellularization and Promote Quiescent Behavior After Monolayer Formation. Front. Bioeng. Biotechnol. 10, 1–15 (2022).
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.