The regulation and remodeling of the tumor microenvironment (TME) are closely associated with how cancers typically progress and metastasize. The extracellular matrix (ECM) is an intricate network of macromolecules that supports cells by controlling major processes like anchorage, migration, and proliferation.
Degradation of the ECM is linked with diseases like cancer, where tumors exploit ECM remodeling to cultivate a microenvironment that encourages tumorigenesis and metastasis. This remodeling gives structural support while impacting cellular pathways, intercellular communication, signal transduction, and cell proliferation and death regulation.1
ECM remodeling involves four key processes: (1) ECM deposition, which modifies component abundance and impacts biochemical and mechanical properties; (2) chemical modification at the post-translational, altering biochemical traits and structure; (3) overcomes cellular barriers via proteolytic degradation and the release of bioactive fragments and factors; and (4) force-mediated physical remodeling, aligning fibers and facilitating cell migration.1
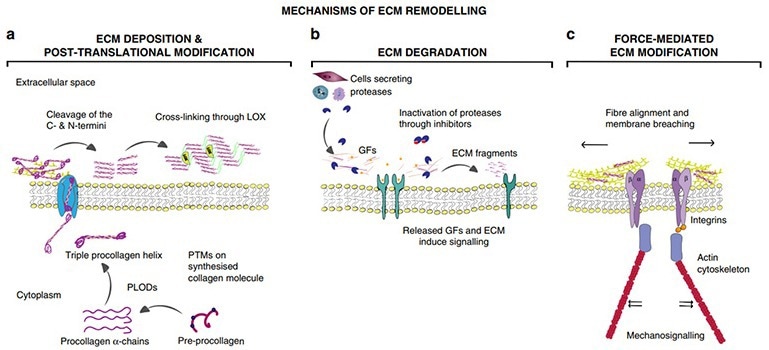
Mechanisms of ECM remodeling. Image Credit: ACROBiosystems
Key role of MMPs in ECM remodeling
Matrix metalloproteinases (MMPs), zinc-dependent endopeptidases, are responsible for the degradation of ECM components during tissue remodeling, playing a key role in ECM dynamics.2
Typically overexpressed in the TME, MMPs cleave the ECM proteins, which discharges Matrix-bound growth factors (GFs) and cytokines, and ECM fragments, such as matrilines and also eliminates any barriers for cell migration, and enables the migration and dissemination of tumor cells.1
There are 23 structurally related MMPs known to be present in humans. These can be classified into six groups based on substrate specificity and structural differences: collagenases, gelatinases, stromelysins, matrilysins, metalloelastase, and membrane-type MMPs.3
The proteolytic degradation of ECM components by MMPs has elaborate, dual effects on tumorigenesis. For example, MMP-8 demonstrates this complexity by both encouraging and restricting tumors. Elevated MMP-8 levels are associated with reduced survival in ovarian and hepatocellular cancers. Its low expression corresponds with poor prognosis in oral tongue squamous cell carcinoma.
These context-dependent outcomes highlight the complex influence of ECM-degrading enzymes on cancer advancement.4-6 The role of MMPs, therefore, have a dual purpose in the TME.
Moreover, it is key to recognize that MMPs’ roles in tumor progression reach beyond the proteolysis of the ECM. For instance, MMP-3 can bind and limit noncanonical Wnt5b, thereby boosting canonical Wnt signaling.7 Likewise, MMP-14 (MT1-MMP) enables basal cell extrusion throughout epithelial-mesenchymal transition (EMT) and drives basal cell mitosis via non-proteolytic mechanisms.
Role of MMPs in cancer
As agents with the capacity to remodel ECM, MMPs have been associated with a wide range of biological processes. The surmounting evidence indicates that heightened MMP expression is closely woven into the fabrics of tumor proliferation, invasion, and metastasis.3
Across a diverse range of cancers, including colorectal, breast, and lung cancers, there has been documentation of significantly elevated MMP expression levels. These elevated MMP levels not only enable the migration and dissemination of tumor cells but also drive tumor growth and angiogenesis by releasing growth factors. Moreover, various members of the MMP family have unique roles.4
- MMP-1 is involved in disease processes like lung emphysema, arthritis, and other cancers, driving cancer cell invasion and migration. Elevated MMP-1 expression has been witnessed in cancers, including oral and bladder, and is associated with a poor prognosis.9
MMP-1 advances colorectal cancer malignancy via EMT and Akt signaling, and its upregulation along with VEGF-C marks poor prognosis in esophageal carcinoma. MMP-1 also triggers other MMPs, such as MMP-9 and MMP-2, and is key in processes like angiogenesis, embryogenesis, morphogenesis, and wound repair.10
- Tumor cells can generate or provoke MMP-2 in neighboring cells, driving tumor progression. MMP-2 corrupts the ECM, promoting cancer cell migration and influencing growth signaling, apoptosis resistance, and angiogenesis.11
Overexpression is frequently observed in cancers like bladder, breast, and lung. MMP-2 is essential in the proteolytic breakdown of ECM components and basement membrane, making it a key target for tissue repair and countering cancer metastasis.
- MMP-3 has dual roles in malignancy, functioning as both a tumor promoter and inhibitor depending on its substrates. It inhibits angiogenesis by degrading plasminogen and type VIII collagen, but it can also promote cancer cell proliferation.
Elevated MMP-3 expression is connected to several cancers, including breast, oral squamous cell, prostate, and non-small-cell lung cancer, signifying its potential as a prognostic factor and therapeutic target.9
- Overexpression of MMP-7 can influence the invasive, metastatic, and angiogenic characteristics of cancer cells, making it a key target for anticancer drug development. Inhibitors that target MMP-7 have demonstrated potentially promising therapeutic effects in preclinical studies for various types of cancer. 9
- MMP-9 is related to immune evasion in hepatocellular carcinoma, and thus, restricting MMP-9 or utilizing anti-MMP-9 monoclonal antibodies can improve the effectiveness of immunotherapy.
In colorectal cancer and adenomas, concentrations of MMP-7, MMP-9, TIMP-1, and TIMP-2 show considerably elevated levels, playing central roles in the development of colorectal cancer.
Upregulation of chemokines CCL27 and CXCL10 can boost the potential invasiveness and migration of cancer cells through activation of specific MMPs like MMP-7 and MMP-9.12
- MMP-14, a key player in EMT, amplifies cell migration and invasion by damaging the ECM. Its role in regulating cell-matrix interactions facilitates the transition from epithelial to mesenchymal states, enhancing migratory capacity.
Overexpression of MMP-14 is related to cancer invasion and metastasis, making it a key regulator in tumor development.
Notably, MMP-14 stimulates other MMPs, including MMP-2 and MMP-9, further advancing ECM degradation and tumor cell invasion.
Its presence acts as a prognostic indicator for several different cancers, corresponding with unfavorable outcomes and cancer spread to distant sites in the body.9
Inhibitors of matrix metalloproteinases
MMPs have been continually investigated over time for their role in cancer progression, migration, and metastasis. Several MMPs have become desirable therapeutic targets for anticancer drug development as a result. Scientists have designed a series of MMP inhibitors. These inhibitors impede the activity or expression levels of MMPs, restricting tumor cell invasion and metastasis.
Some MMP inhibitors are now in clinical trials and have demonstrated potentially good therapeutic effects in certain types of cancer.
A number of drug delivery systems exploiting MMP responsiveness have been explored. Liu et al. contributed to this field by designing a vesicular drug delivery system, ITC⊂N-G-C, which incorporates a polymeric shell cross-linked by MMP-2-degradable peptides.
This design ensures systemic stability and facilitates tumor-specific drug release through MMP-2-mediated shell degradation, thereby improving the therapeutic efficacy in cancer treatment.13
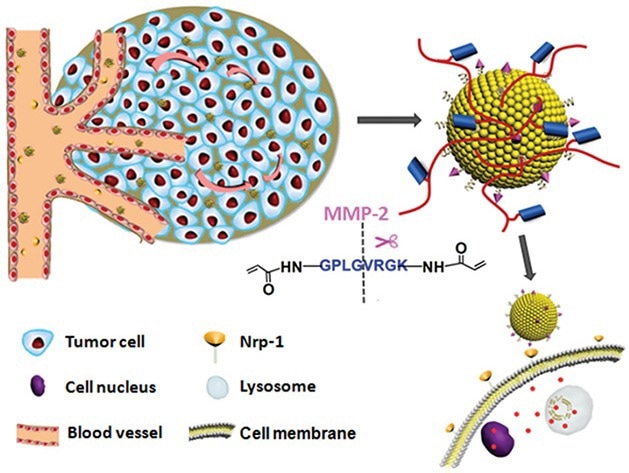
Schematic illustrations of the mechanism of the matrix metalloproteinase (MMPs)-responsive drug‐delivery system (ITC⊂N-G-C) in the tumor microenvironment. Image Credit: ACROBiosystems
The research team, led by Hu et al., developed a gelatin-based multistage drug delivery system with surface-modified nanoparticles (RGD-DOX-DGL-GNP) around 34.3 nm in size. The gelatin core, serving as an MMP-2 substrate, factored in and enabled size reduction from 155.4 nm to smaller dimensions where MMP-2 was present.
In vitro experiments confirmed the reduction of the nanoparticles’ size upon MMP-2 exposure, while tumor penetration tests demonstrated improved deep tumor infiltration capabilities. In vivo assessments underscored the system’s excellent tumor targeting and potent anti-tumor effects.14
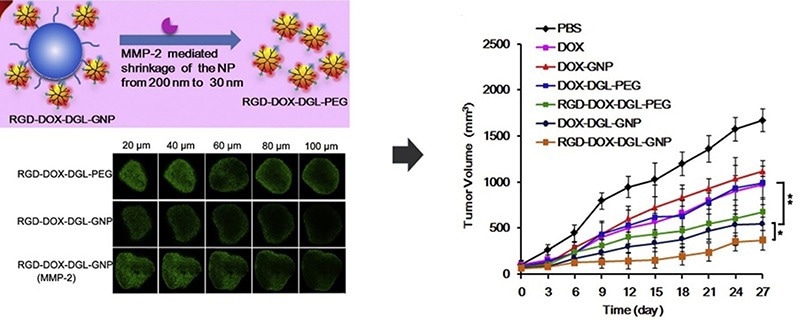
Image Credit: ACROBiosystems
Alongside such developments, novel approaches using nanotechnology have been designed in cancer treatment settings. A nanofiber system that combines DOX with the KGFRWR peptide, a derivative of amyloid ß protein, showed promise in effectively reducing hepatocellular carcinoma growth. Here, DOX’s cytotoxicity targets tumor cells while the KGFRWR peptide restricts MMP activity.15
Similarly, metallofullerenol nanoparticles such as Gd@C82(OH)22 have demonstrated the capacity to inhibit MMP-2 and MMP-9 synthesis via allosteric mechanisms.16
MMPs play a key role in transforming the tumor microenvironment and cancer development. By evaluating the mechanisms of MMP action and designing effective MMP inhibitors, new pathways for cancer treatment can be explored.
High-purity and enzyme-stable MMP proteins for research
Acro Biosystems offers a wide spectrum of purified MMP proteins (>90 % purity validated by SEC-MALS) for a diverse range of applications, such as immunization, antibody screening, immunology, drug hit-lead discovery, and enzyme research
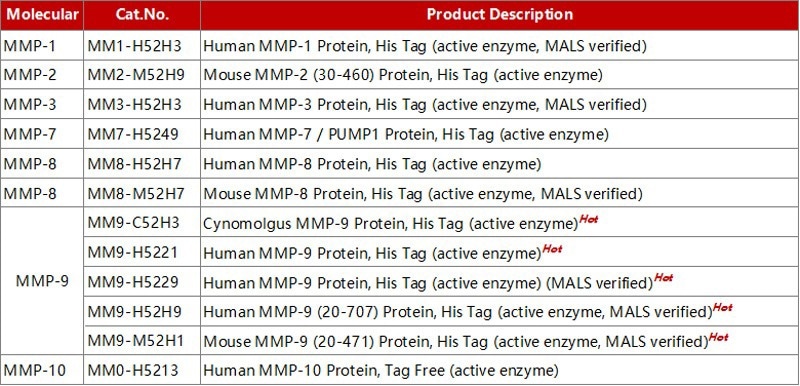
Image Credit: ACROBiosystems
Assay data
• High purity (>90 %) verified by SDS-PAGE
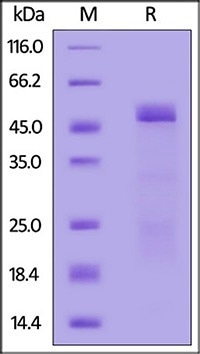
Mouse MMP-2 (30-460) Protein, His Tag (active enzyme) (Cat. No. MM2-M52H9) on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 85 %. Image Credit: ACROBiosystems
• Protein homogeneity and high purity (>90 %) verified by SEC-MALS
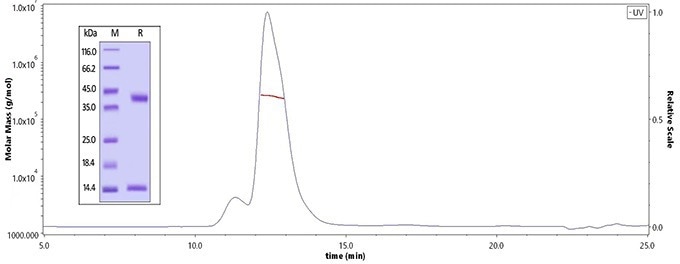
The purity of Human MMP-9, His Tag (Cat. No. MM9-H5229) is more than 90 %, and the molecular weight of this protein is around 50-70 kDa verified by SEC-MALS. The purity of the protein is greater than 95 % verified by SDS-PAGE. Image Credit: ACROBiosystems
• Enzyme activity data
Source: ACROBiosystems
| |
|
|
| Cat. No. |
Human MMP-9 Protein, His Tag (active enzyme) (MALS verified) |
| Substrate |
Mca-PLGL-Dpa-AR-NH2 |
Enzyme Activity
(pmol/min/μg) |
ACRO product
(Cat. No. MM9-H5229) |
Competitor R |
| > 3,000 (QC tested) |
> 1,300 |
Source: ACROBiosystems
| |
|
|
| Cat. No. |
Human MMP-7 / PUMP1 Protein, His Tag (active enzyme) |
| Substrate |
Mca-PLGL-Dpa-AR-NH2 |
Enzyme Activity
(pmol/min/μg) |
ACRO product
(Cat. No. MM9-H5249) |
Competitor R |
| > 600 (QC tested) |
> 600 |
Source: ACROBiosystems
| |
|
|
| Cat. No. |
Human MMP-3 Protein, His Tag (active enzyme, MALS verified) |
| Substrate |
Mca-RPKPVE-Nval-WRK(Dnp)-NH2 |
Enzyme Activity
(pmol/min/μg) |
ACRO product
(Cat. No. MM3-H52H3) |
Competitor R |
| > 422 (QC tested) |
> 150 |
References:
- Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K.J. et al. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 11, 5120 (2020).
- Abdool, A.Y.; Abbas, L.; Sebaei, T.; Schmitt, E.; Sikora, A. How can we design an inhibitor with an enhanced binding affinity that is selective for MMP12? In Protein Modeling Reports 4; NSUWorks: Fort Lauderdale, FL, USA, 2021.
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. S1), 177–183.
- Stadlmann, S. et al. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur. J. Cancer 39, 2499–2505 (2003).
- Qin, G. et al. Reciprocal activation between MMP-8 and TGF-beta1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett. 374, 85–95 (2016).
- Astrom, P. et al. The interplay of matrix metalloproteinase-8, transforming growth factor-beta1 and vascular endothelial growth factor-C cooperatively contributes to the aggressiveness of oral tongue squamous cell carcinoma. Br. J. Cancer 117, 1007–1016 (2017).
- Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
- Andrieu, C. et al. MMP14 is required for delamination of chick neural crest cells independently of its catalytic activity. Development 147, dev183954 (2020).
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.