Imagine the ability to culture a complicated collection of cells that mirrors the cellular tissue environment of the patient and, by extension, organs. Over the last few years, thanks to so-called “organoids” — three-dimensional constructs that copy the functionality and architecture of native organs — this has become a reality.
Organoids consist of self-renewing stem cell populations, which have the potential to differentiate into specific cell types in organ tissues. In comparison to conventional 2D cell culture, organoids possess richer cell composition and physiological function — hence their extensive use in artificial organ development, biological function research, disease modeling, and drug screening.
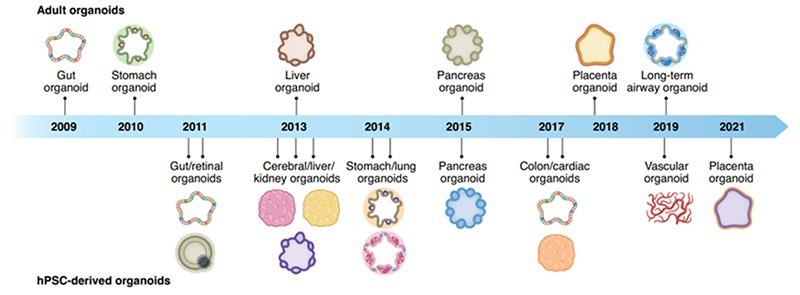
Figure 1. Organoid discovery of their corresponding organs through the years. 1 Image Credit: ACROBiosystems
Organoids sourcing and culturing: Stem cells and growth factors
There are two main sources of organoids: pluripotent stem cells (PSCs) and adult stem cells (ASCs), whereas the former includes embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).
ASCs are classed as cell types that retain stem cell potential in adult human organs, which can promote the homeostatic maintenance or damage repair process of organs within the body.
On the whole, ASCs are organ-specific, and their organoids are precise representations of both functional and structural characteristics of adult tissues. At the same time, PSCs need to experience several differentiation processes.
This stimulates the organ development process, thereby making it a crucial tool in organ development and gene function studies. At present, both stem cell sources have the potential to form the majority of vital organs. However, ASCs are more extensively utilized to build organs and tissues.
There are numerous exceptions for tissue samples that are tougher to obtain, like brain tissue. In such cases, PSCs are considerably more beneficial for organoid model building. In spite of the variations between the stem cell sources, growth factors could play a crucial role in inducing and directing cell differentiation.
Normally, organoid culturing consists of multiple growth factors and could be highly variable between organoid types. Therefore, organoid research needs an improved extracellular matrix composition to guarantee correct physiological development.
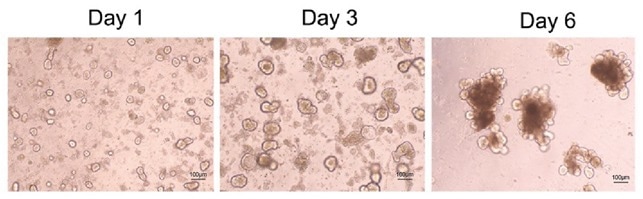
Figure 2. Confocal microscopy of mouse intestinal organoid growth with growth factors: human EGF (Cat. EGF-H52H3), Noggin (Cat. NON-H5257), and R-spondin1 (Cat. RS6-H4220). Image Credit: ACROBiosystems
Application of organoids in research
At present, organoids tend to play a vital role in three primary areas of research: drug discovery, precision medicine, and regenerative medicine. As far as precision medicine is concerned, organoid models are ideal for high-throughput drug screening.
For organoid culturing, a biopsy is taken from a patient, where the cultured organoid is further exposed to several xenobiotics to screen for the optimal therapeutic effect, thereby allowing for personalized medicine.
Organoids have shown themselves to be outstanding drug screening models in a range of tumor types, like breast cancer, colorectal cancer, gastric cancer, non-small cell lung cancer, etc. As per several research articles, the accuracy of organoid drug response prediction in a variety of tumors can reach 80%, which has shown great potential in the implementation of personalized medicine for diagnostic use.
In the field of drug development, organoids tend to constitute a modality that is much more accurate than traditional cell lines and is more affordable than animal models. Its complicated tissue structure, cell composition, and physiological structure, collectively with the high repeatability and throughput of in vitro studies, make it a perfect model for preclinical drug toxicity evaluation.
Consequently, gene editing could be utilized to build a range of disease models, like microcephaly, colorectal cancer, cystic fibrosis, and other diseases. Organoids can also be utilized to learn host-microbial infection processes, like the invasion of Heliobacter pylori and COVID-19.
Over the last few years, as a result of the COVID-19 pandemic, researchers have built a range of organ models to study the infection process and the physiological function changes that are caused by infection.
Similarly, the world’s top 20 pharmaceutical companies, such as Pfizer, Novartis, and Johnson & Johnson, have all taken part in the research and development of organoid applications to test the safety and efficacy of their novel drugs.
As far as regenerative medicine is concerned, organoids, as bigger, more mature stem-cell models, have also been studied to explore the ability to repair damaged tissues and organs.
Numerous studies have also examined the transplantation of organoids to fight against inflammatory bowel disease and treat short bowel syndrome.
Furthermore, numerous studies have been performed to attenuate type I diabetes by building islet organoids. However, they are limited to preclinical animal models, and these studies have yet to be taken to the next stage.
Key problems such as the heterogeneity of cultured organoids, the potential tumorigenicity of using matrix gel in organoid culture, and the homing effect in vivo after organoid transplantation are all concerns when it comes to clinical use. In spite of these problems, organoids' potential in regenerative medicine makes them a focal point in organoids research.
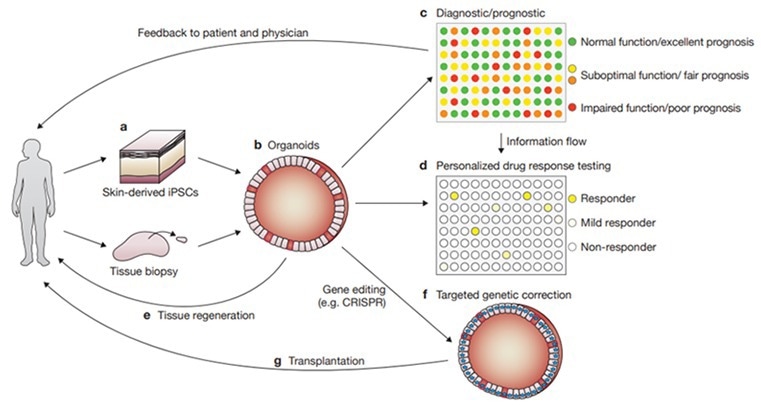
Figure 3. Various applications of organoids for disease research, drug development, and personalized medicine. 2 Image Credit: ACROBiosystems
The future of organoids
Organoids tend to constitute an additional step beyond two-dimensional cell culturing, thereby simulating the functional and architectural characteristics of human organs and the tumor microenvironment.
Having noted the recent progress in organoid culturing and clarification of growth factors required to direct cell differentiation, organoids have become an essential tool in the process of drug development and evaluation.
At present, the majority of the organs in the human body can be developed into organoids in vitro, reproducing blood vessels, the liver, heart, lungs, and many others. Therefore, organoids have the ability to become a necessary preclinical model for human disease research and drug development.
To assist research on organoid cell culturing, ACROBiosystems has come up with a range of high-quality activemax® cytokines, such as EGF, Noggin, R-Spondin 1, FGF2, FGF10, and Activin A. Such products are ideal as growth factors for organoid cultures that have been verified to promote organoid growth.
References
1. Han Y., Chen S., et al. Human Organoid Models to Sutdy SARS-CoV-2 Infection. Nature Methods. 2022, 19,418-428.
2. Fatehullah A., Barker N., et al. Organoids as an In Vitro Model of Human Development and Disease. Nature Cell Biology. 2016, 18(3):246-254.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.