Therapeutic antibodies are highly useful tools in the medical field for the treatment of a range of disorders. They have become one of the leading therapeutic modalities, with more than 50 approved products and more than 500 monoclonal antibody (mAb)-based therapies under clinical development.
Antibodies, or natural macromolecules, exhibit a high affinity and specificity for binding to various antigenic targets through exclusive pharmacodynamic (PD) and pharmacokinetic (PK) properties. The drawbacks of antibodies are mainly due to their large size and poor penetration in solid tissues.
Nearly three decades ago, researchers unexpectedly discovered functional heavy-chain antibodies in the serum of camels (camels, dromedaries, llamas, vicuñas and guanacos). This heavy-chain antibody’s variable domain shows complete antigen-binding potential and powerful affinity with homologous antigens. This makes it the smallest naturally occurring complete antigen-binding fragment.
ABLYNX (acquired by Sanofi in 2018) has developed antibodies with only VHH fragments. These antibodies are based on heavy-chain antibodies. ABLYNX put forward the idea of “Nanobody” due to its small nanoscale size.
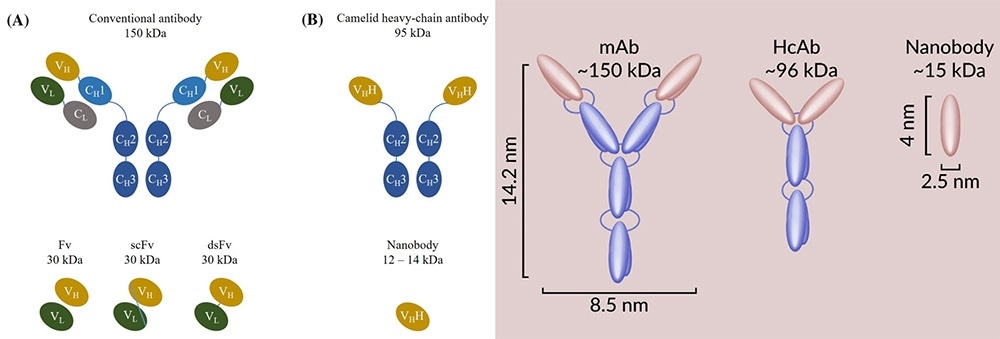
Different structures of antibodies: mAb, HcAb (heavy chain antibody), and Nanobody. Image Credit: ACROBiosystems
The advantages of Nanobody
Nanobody is an innovative, exclusive and complete antigen-binding fragment that features lower molecular weight (around 15 kDa, which is 10 times smaller than monoclonal antibodies), simple structure, good water solubility, optimal physical and chemical stability, ability to penetrate the blood-brain barrier and strong tissue permeability.
It is viable to efficiently synthesize Nanobody using recombinant microorganisms. This can be done at low cost and the Nanobody can be easily assembled or merged into more complex multi-energy structures.
Nanobody combines the benefits of conventional antibodies and small molecular drugs, thus almost overcoming the defects of conventional antibodies with low stability, long development cycles and harsh storage conditions. Nanobody has slowly turned into an emerging force in a new class of therapeutic biomedicine and clinical diagnostic reagents.
Limitations and coping strategies of Nanobody
Nanobody presents a major drawback in that it is quickly cleared in the blood, resulting in a short half-life of the drug. To overcome this issue, the long-acting platform technology of protein drugs can be used to prolong the action duration of these biologics. It involves two key approaches:
- Increasing the hydraulic radius of protein drugs and decreasing the glomerular filtration rate
- Using binding balance, releasing drug and plasma protein and extending the half-life
The latest technologies are based on the above two approaches, including Human Serum Albumin (HSA) fusion, polyethylene glycol (PEG) modification, Fc fusion, etc. PEG modification increases the hydrodynamic radius and extends the half-life of the drug by adding hydrophilic PEG chain molecules. Fc fusion or albumin fusion extends the half-life of the drug through a pH-dependent FcRn recirculation pathway.
Moreover, certain emerging technologies — like XTEN fusion, ABD fusion and a polypeptide segment that binds to albumin — have slowly made it to the front stage. These technologies increase molecular weight by fusing inert proteins. CTP fusion is another technology that introduces glycosylation modification sites.
HSA-fusion Nanobody
HAS, a therapeutic protein, is mainly used for disease diagnosis and drug delivery. HSA-fusion Nanobody has the potential to increase the drug’s half-life and enhance its efficacy.
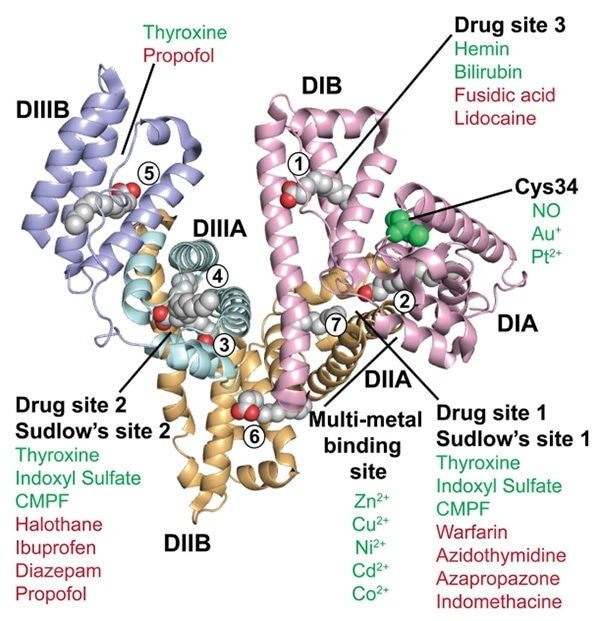
Crystalline structure of HSA. Image Credit: ACROBiosystems
From the X-ray crystal structure of HAS, it can be seen that it is a heart-shaped molecule with 585 amino acids. It lacks β-folding and mainly includes an α-helical structure. It is integrated into three homologous domains — DI, DII and DIII. Each of these domains is divided into A and B subdomains (DIA, DIB, DIIA, DIIB, DIIIA and DIIIB).
Long and flexible rings connect these domains. HSA is the key protein contained in plasma, with a half-life of 20 days and a concentration of around 45 mg/mL (0.6 mm). The unique half-life extension technology from ABLYNX integrates Nanobody with HSA to enhance the molecular weight of certain antibodies. The recirculation effect of FcRn is employed to increase the half-life from a few hours to over three weeks.
Fc-fusion Nanobody
Besides its short half-life, Nanobody does not perform Fc-mediated effector functions because it lacks Fc fragments of antibodies, for example, for anti-tumor therapy. In addition, it lacks the serum stability brought about by Fc and antibody-dependent cell-mediated cytotoxicity (ADCC).
Natural killer (NK) cells are the crucial immune cells inducing ADCC action, and the Fc receptor FcyRIIIa (CD16A) found on the cell surface has a vital role in identifying and binding Fc.
It is simple to equip Nanobody with Fc regions (hinge, CH2, and CH3 domains) in the form of recombinant humanized heavy-chain antibodies to realize effector functions, such as Fc-mediated and increased blood residence time. However, this will obviously decrease its diffusion benefit. Fc-fusion fragments tend to choose IgG1 Fc, which is associated with the affinity binding properties of IgG1 to the Fc receptor proteins.
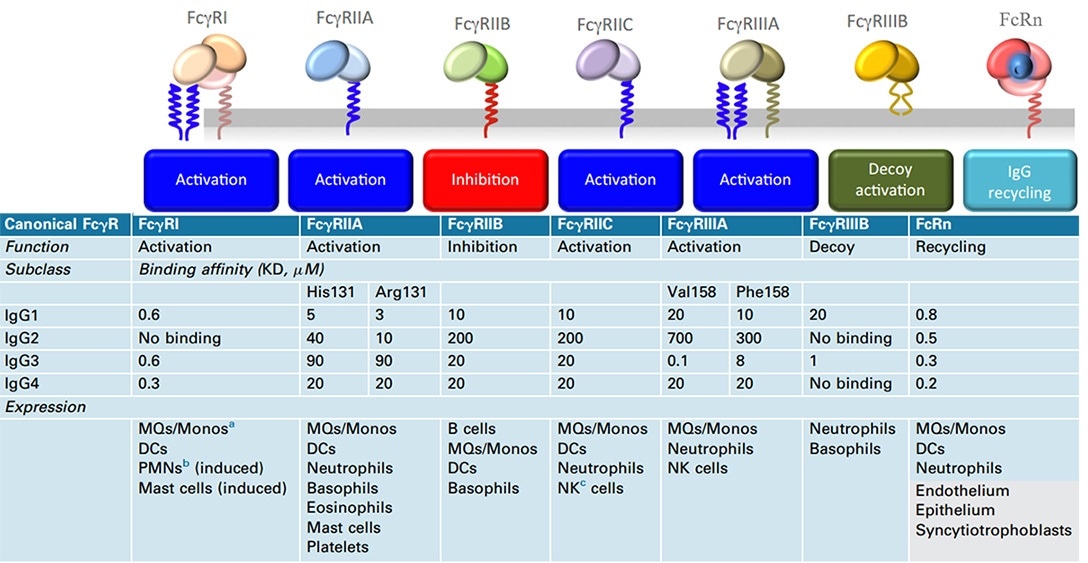
Human canonical FcγR, functions, binding affinities to various IgG subclasses, and expression in cells. Image Credit: ACROBiosystems
The table below illustrates that the Fc-fusion drugs approved by the FDA include the human IgG1 Fc domain, and their main function is to extend the serum half-life of the active part.
Marketed Fc-fusion drugs. Source: ACROBiosystems

Apart from increasing the half-life, Fc-fusion also enhances the solubility and stability of the drug. It is advantageous for the production process because Fc’s fusion expression is conducive to the affinity purification of protein A. This simplifies the downstream purification process of protein drugs.
Thus, Fc-fusion proteins have realized major success in terms of the number of approved products, global value and applications in various disease fields.
Summary
Antibodies are the key macromolecules applied in targeted therapy, with the ability to considerably enhance the clinical care and quality of life of cancer patients. Yet, the drawbacks of antibodies — such as their size, possible immunogenicity and incomplete tumor penetration — have resulted in the development of a new class of small drugs.
Besides offering new possibilities for the treatment of cancer, Nanobody also offers new therapeutic possibilities for different human diseases at the subcellular level, which will completely transform the field of biomedicine.
Thanks to its good tissue permeability and small size, Nanobody might be the perfect diagnostic and therapeutic tool. But the lack of functional Fc makes the half-life very short and the clearance rate in the kidneys very quick.
Fc and HSA fusion technologies bestow more antibody-like characteristics to peptides and proteins of therapeutic value. At present, Nanobodies are used extensively in clinical development and treatment fields such as hematology, inflammation and infectious diseases.
Prolonged exposure to Nanobody is vital for indications of chronic diseases. The structure prolonging its half-life can be generated by fusing Fc or HSA. Non-half-life-prolonging variants may exhibit more robust kinetic benefits in acute indications.
Moreover, the introduction of anti-FcR or anti-HSA to Nanobody to mediate the binding of the drug to HSA can also considerably increase the half-life of the drug. Researchers have reported a range of bispecific Nanobodies based on anti-HAS, anti-tumor antigen and anti-CD16 Nanobodies. Without a doubt, reasonable drug design can help Nanobody to exhibit its complete potential.
ACROBiosystems offers the following range of products to support the development of Nanobody drug:
- Hot target proteins, ideal for screening, immunization, process method development and optimization
- HSA and IgG Fc feature homogeneous structure, strict standards and verified activity to fulfill the development of Fc-fusion and HSA-fusion products, specifically in the process of method development and functional verification for isotype control requirements. HSA can serve as a binding ligand while developing anti-HSA Nanobody drugs.
- A complete range of high-quality Fc receptor proteins: FcRn, FcγRI/CD64, FcγRIIIa/CD16A, FcγRIIA/CD32a, FcγRIIIa/CD16B, FcγIIB/CD32B, ideal for validating the half-life and binding affinity of Nanobody drug fused with Fc fragments to Fc receptor proteins.
Serum albumin (SA): Product list
Source: ACROBiosystems
| Molecule |
Cat. No. |
Species |
Product Description |
| Serum Albumin |
HSA-H5220 |
Human |
Human Serum Albumin Protein, His Tag (HPLC verified) |
| Serum Albumin |
HSA-H82E3 |
Human |
Biotinylated Human Serum Albumin Protein, His, Avitag™ |
| Serum Albumin |
HSA-H522a |
Human |
Human Serum Albumin Protein, His Tag, low endotoxin |
| Serum Albumin |
MSA-M52H8 |
Mouse |
Mouse Serum Albumin Protein, His Tag |
| Serum Albumin |
MSA-M82E4 |
Mouse |
Biotinylated Mouse Serum Albumin Protein, His, Avitag™ (MALS verified) |
| Serum Albumin |
CSA-C52H4 |
Cynomolgus |
Cynomolgus Serum Albumin Protein, His Tag |
| Serum Albumin |
HSA-C82E5 |
Cynomolgus |
Biotinylated Cynomolgus Serum Albumin Protein, His, Avitag™ |
| Serum Albumin |
RSA-R52H6 |
Rabbit |
Rabbit Serum Albumin Protein, His Tag |
Serum albumin (SA): Product advantages
- The SA sequence is a fully natural sequence including only the signal peptide and propeptide eliminated
- Different species: Human, Rabbit, Mouse, Cynomolgus
- High purity: over 95% as validated by SDS-PAGE and over 90% as validated by SEC-MALS
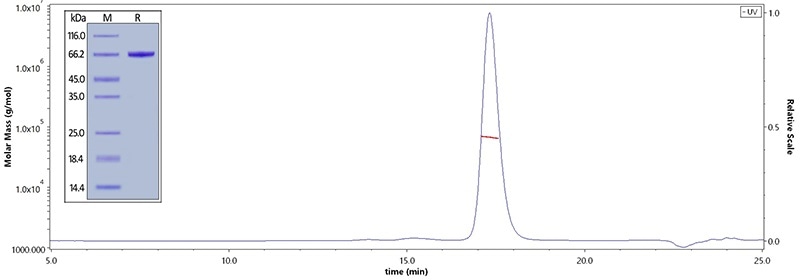
Human Serum Albumin, His Tag (Cat. No. HSA-H5220) on SDS-PAGE under reducing (R) condition. The purity of the protein is greater than 95%. The purity of Human Serum Albumin, His Tag (Cat. No. HSA-H5220) was more than 90% and the molecular weight of this protein is around 60–75 kDa verified by SEC-MALS. Image Credit: ACROBiosystems
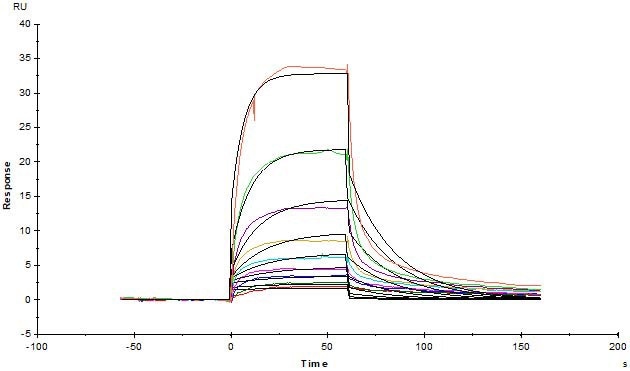
Human Serum Albumin Protein, His Tag (Cat. No. HSA-H5220) immobilized on CM4 Chip can bind Human FCGRT&B2M Heterodimer Protein, His Tag (Cat. No. FCN-H52W7) with an affinity constant of 0.381 μM as determined in an SPR assay (Biacore T200). Image Credit: ACROBiosystems
IgG Fc: Structure and application
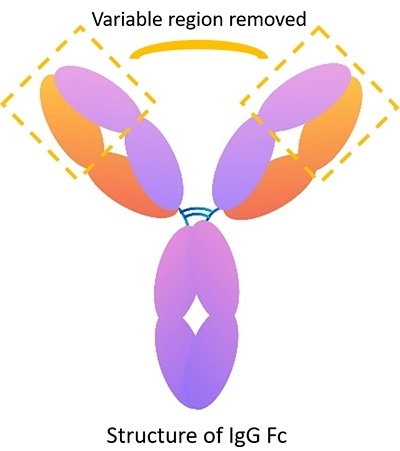
Image Credit: ACROBiosystems
What can IgG Fc be used for?
IgG Fc is an isotype control of IgG subtype antibody that can be used for functional verification and antibody screening.
How does IgG Fc realize the effect of isotype control?
The IgG Fc recombinant protein includes constant region sequences, such as hinge region, CH1, CH2, CH3. However, it does not include sequences of the variable region. Therefore, it can be used as a control to verify the particular role of an antibody in identifying the target antigen. For instance, if the IgG Fc isotype control group’s result is negative, the experimental group’s result accurately shows the effect of the antibody’s variable region.
IgG Fc: Product list
Source: ACROBiosystems
| Molecule |
Cat. No. |
Species |
Product Description |
| IgG1 Fc |
FCC-H5214 |
Human |
Human IgG1 Fc Protein, Tag Free (MALS verified) |
| IgG1 Fc |
IG1-H8213 |
Human |
Biotinylated Human IgG1 Fc protein, Avitag™ (MALS verified) |
| IgG1 Fc |
IG1-H5225 |
Human |
Human IgG1 Fc Protein, His Tag (MALS verified) |
| IgG1 Fc |
IG1-H52C9 |
Human |
Human IgG1 Fc Protein, Flag Tag (MALS verified) |
| IgG1 Fc |
IG1-H52G6 |
Human |
Human IgG1 Fc Protein, gD Tag (MALS verified) |
| IgG1 Fc |
IG1-H82Eb |
Human |
Biotinylated Human IgG1 Fc Protein, Avitag™, His Tag (MALS verified) |
| IgG2 Fc |
IG2-H5206 |
Human |
Human IgG2 Fc Protein, Tag Free (SPR verified) |
| IgG3 Fc |
IG3-H5200 |
Human |
Human IgG3 Fc Protein, Tag Free (SPR verified) |
| IgG4 Fc |
IG4-H5205 |
Human |
Human IgG4 Fc Protein, Tag Free (MALS & SPR verified) |
| IgG1 Fc |
IG1-M5208 |
Mouse |
Mouse IgG1 Fc Protein, Tag Free (HPLC verified) |
| IgG1 Fc |
IG1-M8211 |
Mouse |
Biotinylated Mouse IgG1 Fc protein, Avitag™ |
| IgG2a Fc |
IGA-M5207 |
Mouse |
Mouse IgG2a Fc Protein, Tag Free (MALS verified) |
| IgG2a Fc |
IGA-M8210 |
Mouse |
Biotinylated Mouse IgG2a Fc Protein, Avitag™ (MALS verified) |
| IgG2b Fc |
IGB-M5203 |
Mouse |
Mouse IgG2b Fc Protein, Tag Free (MALS verified) |
| IgG2b Fc |
IGB-L5204 |
Llama |
Llama IgG2b Fc Protein, Tag Free (MALS verified) |
| IgG Fc |
IGG-R5203 |
Rabbit |
Rabbit IgG Fc Protein, Tag Free (MALS verified) |
IgG Fc: Product advantage
- Additional product options: Human IgG1 Fc, IgG2 Fc, IgG3 Fc, IgG4 Fc; Rabbit IgG Fc; Llama IgG2b Fc; Mouse IgG1 Fc, IgG2a Fc, IgG2b Fc
- Expressed by HEK293 cell that achieves post-translational glycosylation as well as other modifications and accurate protein folding
- Different tags to fulfill the requirements of different applications: AvitagTM, Flag tag, Tag Free, AvitagTM & His tag, His tag and gD tag
- High purity of over 95% as validated by SDS-PAGE and over 95% as validated by SEC-MALS
- Low endotoxin lower than 1.0 EU/μg by the LAL method
- Bioactivity validated by ELISA and SPR
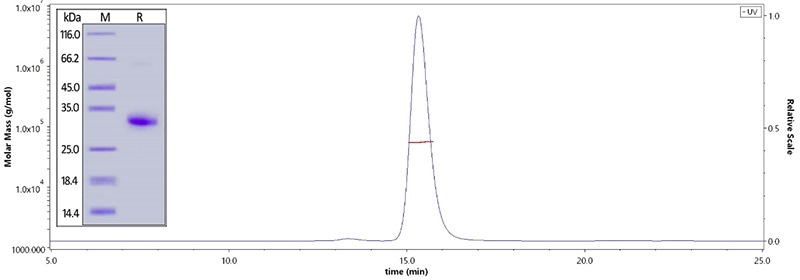
Human IgG Fc, Tag Free (Cat. No. FCC-H5214) on SDS-PAGE under reducing (R) condition. The purity of the protein is greater than 95%. The purity is more than 95% and the molecular weight of this protein is around 51–65 kDa verified by SEC-MALS. Image Credit: ACROBiosystems
- Bioactivity validated by ELISA and SPR
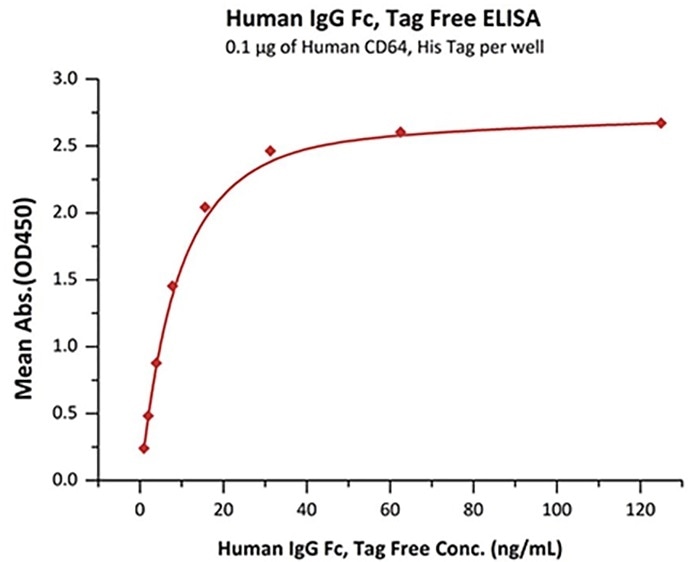
Immobilized Human CD64, His Tag (Cat. No. FCA-H52H1) at 1 μg/mL (100 μL/well) can bind Human IgG Fc, Tag Free (Cat. No. FCC-H5214) with a linear range of 1–16 ng/mL. Image Credit: ACROBiosystems
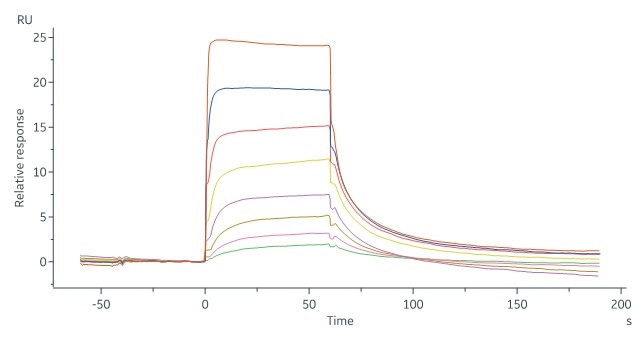
Immobilized Human CD64, His Tag (Cat. No. FCA-H52H1) at 1 μg/mL (100 μL/well) can bind Human IgG Fc, Tag Free (Cat. No. FCC-H5214) with a linear range of 1–16 ng/mL. Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.