Global understanding of complement has shifted and developed since the 21st century: from our idea of a blood-based antibiotic system to a global regulator of immunity and tissue homeostasis. Recent years have ushered in truly revolutionary advances in the mechanism, structure and function of complement activation, which have added new complexity to complement biology.
A wide range of neurodegenerative, inflammatory diseases and cancers reflect this complexity - places where complement affects immunity and disease pathogenesis in a network.
The three activation pathways of the complement and the wider complement system
Also known as the complement cascade, the complement system plays a crucial role in defense against microbial infection and clearance of immune complexes and injured cells and is a crucial part of the innate immune system. Complement, under normal conditions, is tightly controlled by several humoral and cell surface proteins to avoid damage to autologous tissue.
Severe inflammatory responses are triggered in many organs when complement is overactivated in subjects with autoimmune diseases or dysregulated regulatory proteins.
Four distinct categories can be created from the intrinsic components of complement:
- In the classical activation pathway: C1, C2, C4
- In the alternative activation pathway: Factors B, D and P
- In the mannan-binding lectin-activated pathway: MBL and serine protease
- In the common terminal pathway: C3, C5, C6, C7, C8 and C9
Currently, the complement system is known to be made up of more than 30 membrane-bound proteins, soluble proteins, and complement receptors, which are widely distributed throughout tissues and circulation. Under various stimuli, they are synthesized and secreted by many cells, including cytokines and hormones.
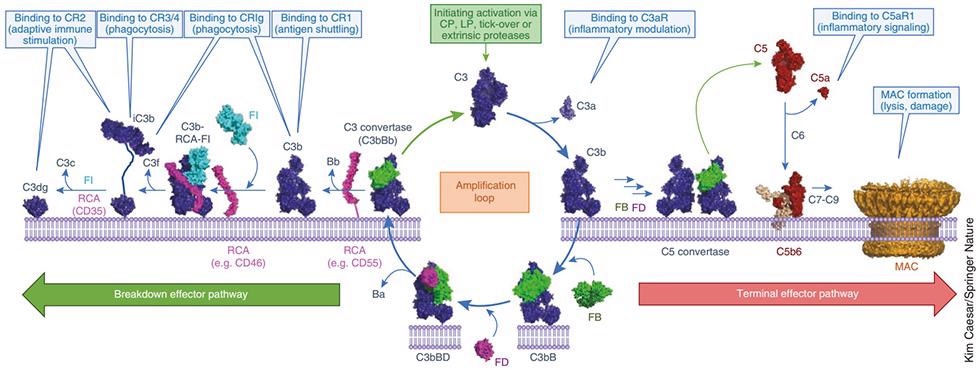
This image displays the molecular mechanism of C3-mediated complement activation, amplification, and effector molecule production. Image Credit: ACROBiosystems
Complement activates three pathways, despite the lack of specificity of the components of the acquired immune system: the lectin pathway, the classical pathway, and the alternative activation pathway, the activation of which enables selective identification of foreign pathogens and damaged endogenous cells.
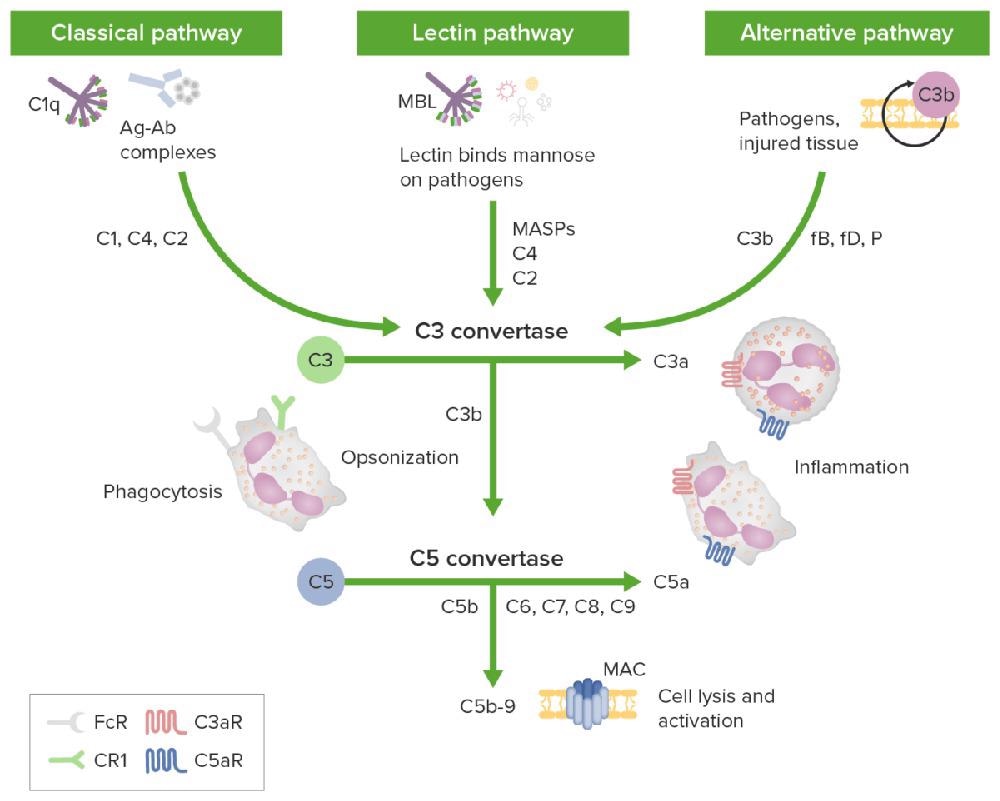
The complement cascade’s three activation pathways. Image Credit: ACROBiosystems
- The classic pathway is triggered by an antigen-antibody complex. At this stage, the inactive C1 circulates in the form of a complex of serum molecules, which includes two serine protease molecules, C1r and C1s and Hexa-C1q molecules. The Fc fragment of an IgG or IgM antibody interacts with the collagen-like tail of C1q after binding to the antigen.
This interaction creates a conformational change along with the sequential activation of C1r, C1s, C4, C2, and C3, which generates a cascade enzymatic reaction process of C3 convertase (C4b2b) and C5 convertase (C4b2b3b).
- The lectin pathway is recognized by mannan-binding lectin glycans, generating the same downstream effect as in the classical pathway.
- A mechanism independent of antibodies triggers the alternative pathway, which involves factors B, D, and P, and directly activates C3 by microorganisms or foreign substances. This forms C3 and C5 convertases, and activates the complement cascade enzymatic reaction.
A common terminal pathway is shared by the above three complement pathways: Under the action of C5 convertase, C5 can be bifurcated into C5a and C5b. C5a, the strongest factor, appears in the early stage of tissue damage inflammation and acute infection and is recognized as a broad-spectrum inflammatory amplifier; a membrane attack complex (MAC, C5b-9) can be formed by C5b with complement proteins C6, C7, C8, and C9. This directly lyses targeted pathogens or damaged autologous cells.
The role of complement in inflammation
The complement system, as part of the innate immune system, is not adaptive throughout individual life: though it has homeostatic importance in early development, in an aging immune system, it is abnormally reactivated. Complement is, therefore, a clear and key contributor to the pathological inflammatory process, which includes age-related diseases.
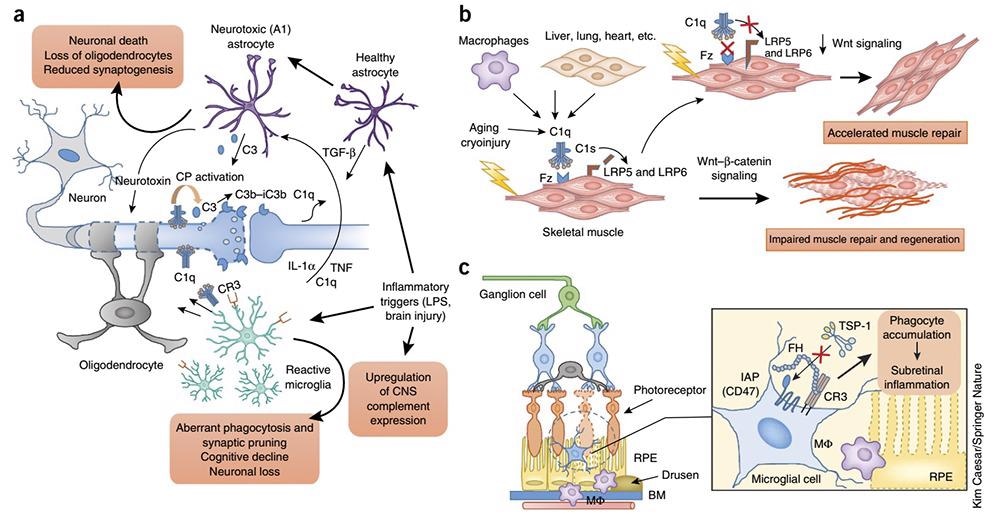
Complement’s role in inflammation. Image Credit: ACROBiosystems
- It has been revealed by studies that complement both drives and participates in inflammatory conditions which are chronic and age-related: this ranges from those affecting the eye, skin, and kidneys to neuroinflammation and neurodegenerative diseases.
- In the cortex of patients with refractory epilepsy, the enrichment of C1q and C3 correlates with abnormal microglial activity markers. It may be possible to attribute the loss of inhibitory synapses to the neuronal hyperexcitability observed in humans with epilepsy.
- The leading cause of age-related dementia worldwide is Alzheimer’s disease (AD), which is characterized by the strong activation of astrocytes and microglia along with the deposition of complement on amyloid β plaques and tangles.
In the phagocytic clearance of amyloid fibrils, late complement components (C1q and C3) have been implicated, whilst late complement effectors (C5a and MAC) have been implicated in inflammatory neuronal damage.
Targeting C5aR1 improved pathological indicators in various transgenic AD models, which is consistent with the above. In addition, within transgenic models of age-related AD pathology, C3 deficiency attenuated synaptic loss and cognitive decline. (Three hypotheses on the pathogenesis of Alzheimer’s disease).
- AMD, or age-related macular degeneration, is a well-known and common ocular inflammatory disease that is currently the leading cause of blindness in the elderly worldwide. FH polymorphisms have been identified in genome-wide association studies as key susceptibility factors for AMD. It has been shown that the binding of FH to CR3 inhibits the activation of the integrin-related receptor CD47 by thrombospondin-1, which is needed for the homeostatic elimination of subretinal phagocytes.
This inhibitory effect is enhanced by the AMD-associated FH variant H402Y, resulting in a pathological accumulation of subretinal phagocytes.
Cancer’s role in complement
A key role is played by complement in determining tumor growth. This occurs by the regulation of T-cell immunity, inflammation, migration, vascularization and tumor cell proliferation and invasive capabilities.
Within the tumor microenvironment, imbalanced complement activation triggers the release of pro-inflammatory cytokines from both tumor cells and tumor-infiltrating immune cells. Local inflammation suppresses the activation of effector T cells, creating an environment that is conducive to - and facilitates - tumor growth.
C5a, among other complement activation products, promotes angiogenesis and tumor cell migration, along with the invasion and metastasis of adjacent tissues.
It has been proven that complement regulates the differentiation and recruitment of myeloid-derived suppressor cells to tumor sites.
It has also been demonstrated that in animal models of intestinal, cervical, and lung cancers along with melanoma that C3aR and C5ar1 mediated pathways form pro-tumor through the means of activation and polarization of innate immune cells, the inhibition of effector T cells environment and the release of pro-tumor factors.
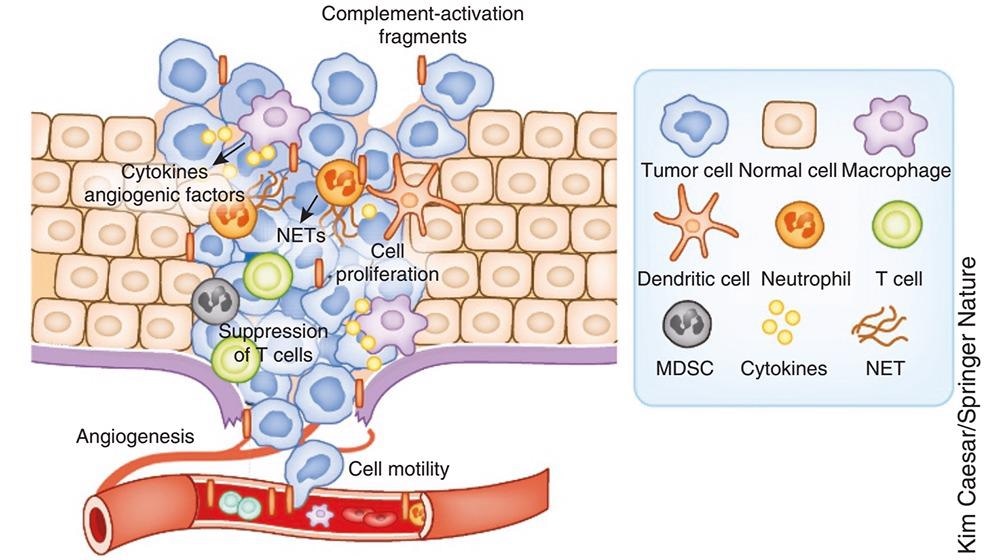
Diagram depicting the role of complement in cancer. Image Credit: ACROBiosystems
The combined blockade of complement and PD-L1 attenuated tumorigenesis more effectively than treatment alone in melanoma and lung cancer models, which opens a new avenue for exploring complement inhibition joined with specific immunotherapy to activate the anticancer immunity of patients.
Furthermore, various monoclonal antibodies used in a clinic setting for cancer treatment perform the function of activating the complement on the surface of tumor cells, which then enhances their killing and elimination effects.
The of complement in COVID-19
A component of the innate immune response, complement, has a pro-inflammatory response to viruses. SARS-CoV-2 studies have discovered that activating complement component C3 can lead to the exacerbation of acute respiratory distress syndrome (ARDS) and that this C3 inhibition may also lessen the inflammatory pulmonary complications of SARS-CoV-2 infection.
Simultaneously, C3 inhibition can block the production of C3a and C5a, along with C3 activation and IL-6 release in the lungs of alveolar macrophages or other cells expressing C3a receptors (C3aRs) and/or C5a receptors (C5aRs). This thereby eases lung injury, as well as suggests the possibility of combining anti-IL-6 regimens with C3 inhibitors.
Patients with severe COVID-19 showed extensive complement activation from lung biopsy samples, which is characterized by C3a production and C3 fragmentation deposition, and markedly increased serum levels of C5a.
Patients who were treated with anti-C5a antibodies displayed immediate clinical improvement. (Therapeutic potential of IL-6 immunization in multiple fields continues to burst)
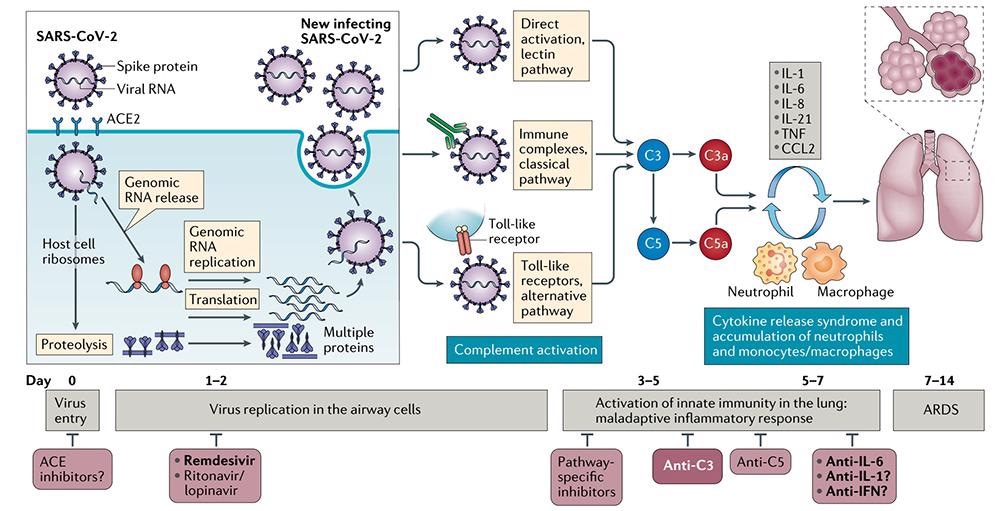
This depicts the role of targeted complement in lung injury associated with SARS-CoV-2. Image Credit: ACROBiosystems
Maladaptive inflammatory responses may be a natural result of complement activation in some severely ill COVID-19 patients. To control ARDS and systemic inflammation in the kidney, brain, microvascular bed, and other vital organs in these severe cases of COVID-19, C3 inhibition has the potential to be broadly applied. C3 or C5 may therefore have strong therapeutic potential.
Our complement-based understanding will be greatly ameliorated by a better understanding of the complement’s structure, function, and biological role, improving treatments for inflammation, cancer, neurodegenerative diseases, and COVID-19.
A series of recombinant complement proteins expressed by HEK293 Cells, including C2, C3, C5, C5a and complement factor D (CFD), has been developed by ACROBiosystems.
Features of product
- Various species
- High purity verified by SDS-PAGE
- Homogeneous structure verified by MALS
- High biological activity verified by ELISA and cell-based assay
- Product list
Product list
| Molecule |
Cat. No. |
Species |
Product Description |
| Complement C2 |
CO2-H52H7 |
Human |
Human Complement C2 Protein, His Tag (MALS verified) (active enzyme) |
| Complement C3 |
CO3-H52H3 |
Human |
Human Complement C3 Protein, His Tag (MALS verified) |
| CO3-M52H4 |
Mouse |
Mouse Complement C3 Protein, His Tag (MALS verified) |
| CO3-C52H5 |
Cynomolgus |
Cynomolgus Complement C3 Protein, His Tag (MALS verified) |
| Complement C5 |
CO5-H52Ha |
Human |
Human Complement C5 Protein, His Tag |
| CO5-H52Hx |
Human |
Human Complement C5 (R885H) Protein, His Tag |
| CO5-H52H7 |
Human |
Human Complement C5 (w917s) Protein, His Tag |
| CO5-M52H4 |
Mouse |
Mouse Complement C5 Protein, His Tag |
| CO5-R52H5 |
Rat |
Rat Complement C5 Protein, His Tag |
| CO5-C52Hx |
Cynomolgus |
Cynomolgus Complement C5 Protein, His Tag |
| CO5-R52H4 |
Rabbit |
Rabbit Complement C5 Protein, His Tag |
| Complement C5a |
C5A-H5116 |
Human |
Human Complement C5a Protein, Tag Free |
| C5A-H525a |
Human |
Human Complement C5a Protein, Fc Tag (MALS verified) |
| C5A-M51H4 |
Mouse |
Mouse Complement C5a Protein, His Tag |
| C5A-C5118 |
Cynomolgus |
Cynomolgus Complement C5a Protein, Tag Free |
| Complement Factor D |
CFD-H52H8 |
Human |
Human Complement Factor D / CFD Protein, His Tag (active enzyme) |
| CFD-H5256 |
Human |
Human Complement Factor D / CFD Protein, Fc Tag |
| CFD-R52H3 |
Rhesus macaque / Cynomolgus |
Rhesus macaque / Cynomolgus Complement Factor D / CFD Protein, His Tag (active enzyme) |
| CFD-R5255 |
Rhesus macaque |
Rhesus macaque Complement Factor D / CFD Protein, Fc Tag |
Partial verification data
High purity>95%(SDS-PAGE)
Protocol
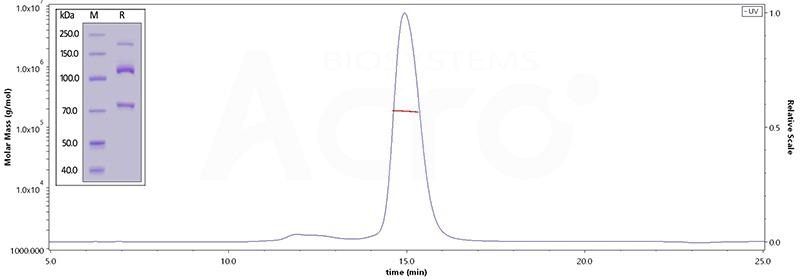
Image Credit: ACROBiosystems
This graph depicts Human Complement C3, His Tag (Cat. No. CO3-H52H3) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%. The purity of Human Complement component 3, His Tag (Cat. No. CO3-H52H3), is more than 90%, and the molecular weight of this protein is around 168-204 kDa, verified by SEC-MALS.
High bioactivity which is verified by ELISA
Protocol
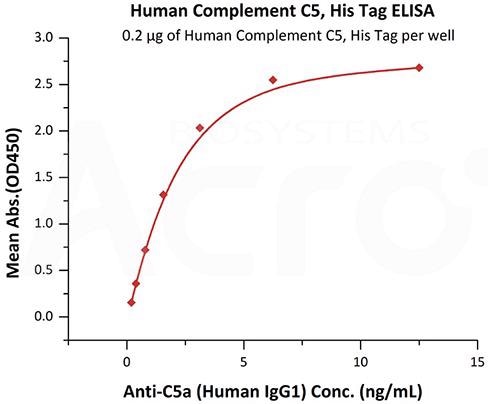
Image Credit: ACROBiosystems
This graph depicts Immobilized Human Complement C5, His Tag (Cat. No. CO5-H52Ha) at 2 μg/mL (100 μL/well) can bind Anti-C5a (Human IgG1) with a linear range of 0.2-3 ng/mL (QC tested).
Protocol
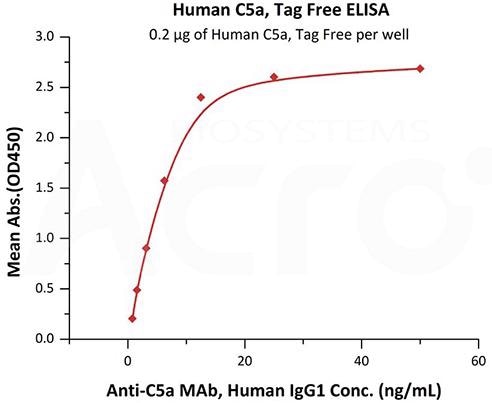
Image Credit: ACROBiosystems
This diagram displays immobilized Human Complement C5a, Tag Free (Cat. No. C5A-H5116) at 2 μg/mL (100 μL/well) can bind Anti-C5a MAb, Human IgG1 with a linear range of 0.8-13 ng/mL (QC tested).
High bioactivity which is verified by cell-based assay
Protocol
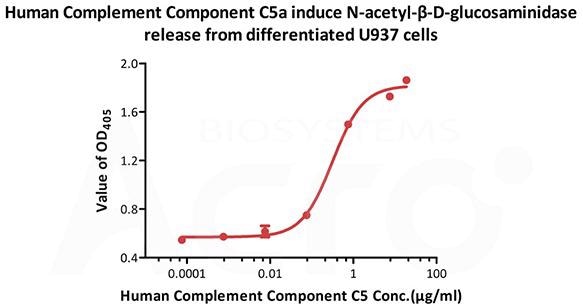
Image Credit: ACROBiosystems
This graph displays Human Complement C5a, Tag Free (Cat. No. C5A-H5116) induce N-acetyl-β-D-glucosaminidase release from differentiated U937 cells. The ED50 for this effect is 0.215-0.323 μg/mL.
A series of recombinant cytokines and their receptors is provided by ACROBiosystems, which include growth factors, interleukins, chemokines, tumor necrosis factors (TNFs), interferons (IFNs) and colony-stimulating factors (CSFs), complement, etc. to improve scientific research and drug development programs.

Image Credit: ACROBiosystems
References
- Hajishengallis, G., Reis, E., Mastellos, D. et al. Novel mechanisms and functions of complement. Nat Immunol 18, 1288–1298 (2017). https://doi.org/10.1038/ni.3858
- https://www.lecturio.com/concepts/innate-immune-response/
- Marina Noris, Giuseppe Remuzzi, Overview of Complement Activation and Regulation, Seminars in Nephrology (2013). https://doi.org/10.1016/j.semnephrol.2013.08.001.
- Risitano, A.M., Mastellos, D.C., Huber-Lang, M. et al. Complement as a target in COVID-19?. Nat Rev Immunol 20, 343–344 (2020). https://doi.org/10.1038/s41577-020-0320-7
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.