Dendritic cells (DCs) are exceptionally potent antigen-presenting cells (APCs) in the human body and are considered the “sentinels” of the immune system. They are essential in establishing, maintaining, and strengthening innate and adaptive immune responses. They interact with various immune cells like T and B cells, increasing the efficacy of immune responses.
DC vaccines have become a topic of interest in cancer immunotherapy with the emergence of the uncommon physiological benefits of DCs. DC vaccines based on development strategies are loosely categorized into three types:
- Early-stage DC vaccines, which utilize antigens in protein or long peptides combined with additives to enhance DC maturation.
- In vivo approach DC vaccines.
- Ex vivo DC vaccines load DCs with antigens ex vivo before reintroducing them into the body. This technique, also called DC cell therapy, has proven promising in preventing and treating various infectious diseases and malignancies.
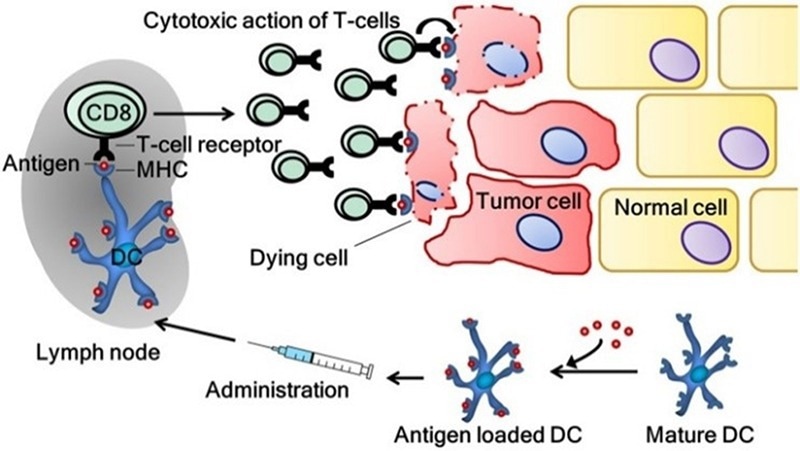
The induction of a tumor-specific immune response by dendritic cell vaccination. Image Credit: Frontiers in Immunology, 9, 2265
DC cell therapy: An ex vivo approach for DC vaccination
In this method, monocytes are first isolated from the patient's blood, or CD34+ precursor cells are collected from the bone marrow. Cells are then stimulated and differentiated using a variety of cytokines, prompting the creation of immature dendritic cells (DCs). These immature DCs are filled with antigens, including nucleic acids to peptides, proteins, cells, or nanoparticles, using methods like cell fusion, co-culture, or transfection.
Further activation encourages maturation, resulting in mature DCs ready to be reintroduced into the patient's body. When reintroduced, the mature DCs process and present the antigens to the immune system. The body’s immune system initiates or increases the response to the targeted antigens.
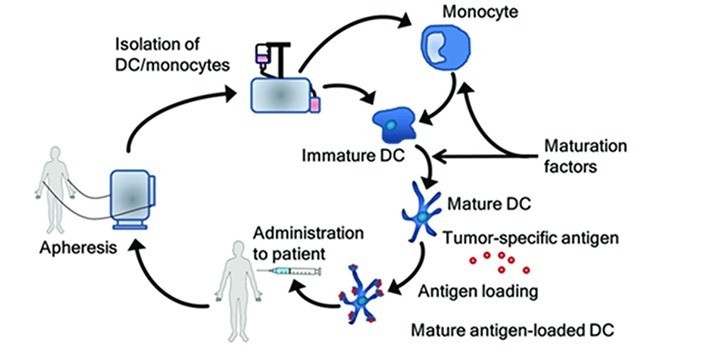
The process of generating dendritic cell vaccines. Image Credit: Frontiers in Immunology, 9, 2265
Ex vivo culturing of DCs is a highly time-consuming step in producing DC vaccines. It requires strict procedures and accurately controlled conditions. The maturity and differentiation of DCs are essential for DC vaccine production. Throughout clinical production, the differentiation and maturation of DCs happens in a mixture of cytokine-rich culture conditions.
GM-CSF, IL-4, and TNF-α growth factors are recognized as the three major growth factors for DC maturation and differentiation. GM-CSF enables DC generation. IL-4 restricts the differentiation of DC precursor cells into CD14+ macrophages, engineering its differentiation into CD14+-CD1a+- DCs.
However, growth factors, including TNF-α, IFN-β, IFN-γ, IL-6, and PGE2, are required to prompt differentiation into mature CD1a+CD83+ DCs for use in the following cell therapy production processes.
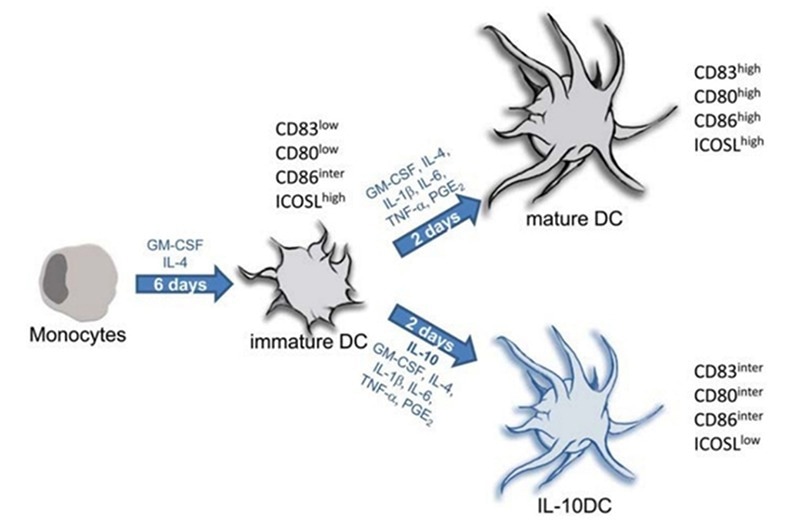
Protocols for generation of tolerogenic and immunogenic dendritic cells from monocytic precursors. Image Credit: Frontiers in Immunology, 4, 82
Approved and current clinical trials of dendritic cell vaccines
Cancer treatments are being revolutionized by DC vaccines that utilize the body's immune system to target cancer cells. Sipuleucel-T (Provenge) is the only FDA-approved DC vaccine for metastatic castrate-resistant prostate cancer. However, other DC vaccines have received approval worldwide for various cancers. Several examples are HybriCell in Brazil, CreaVax-RCC in Korea, and APCEDEN in India.
In addition, DCVax-L has shown promise for treating glioblastoma in Germany. These vaccines use a patient's DCs, filled with tumor antigens, to provoke an immune response against cancer.
Extensive research in DC vaccines has over 300 clinical trials investigating their use in various malignancies. These trials explore techniques to improve vaccine effectiveness, including utilizing cytokine cocktails and integrative treatments with chemotherapy or immune checkpoint inhibitors. This approach offers immense promise for improved cancer treatment outcomes by providing targeted and personalized vaccines customized to individual patients.
Summary
DC is an adaptable instigator with the capability to activate naive T cells, formulating them into a vital part of the body's immune system.
Research in the last two decades on DC vaccines/cell therapy has taken extraordinary steps in improving the migratory abilities and immune effectiveness of DCs.
Understanding the limitations of DC subsets, disease mechanisms, functions, and targets will require a deeper investigation of foundational advances in DC vaccines.
An increasing number of DC vaccines are in clinical trials, recognizing that DC cell immunotherapy will continue to have an increased role in future cancer treatments.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.