Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein found on the cell membrane. PSMA is specifically and highly expressed in prostate cancer (PCa). Its expression level is also related to the invasiveness of a tumor.
PSMA is also a molecular target of PCa, prompting considerable research over the past two decades, not least because prostate cancer remains one of the most common malignant tumors in the United States and Europe.
As China’s economic level improved and its residents’ lifestyles shifted, there was an increased incidence of prostate cancer in the country.
PSMA is highly expressed in advanced and often castration-resistant prostate cancers, but its sizeable extracellular domain does make this an ideal target for immune methods.
Results of PSMA-targeted radionuclide therapy have also verified its potential as an ideal target for diagnosing and treating prostate cancer.
Androgen deprivation therapy (ADT) remains the first-line therapy for treating advanced prostate cancer, but this type of cancer can often develop metastatic or castration resistance, in turn, developing into Castrate-Resistant Prostate Cancer (CRPC).
Androgen receptor blockers (AR) such as enzalutamide and abiraterone have been widely used in clinical practice for the treatment of CRPC, but these therapies involve a limited remission rate.
It is clear that more therapies for PSMA should be explored, and there is a substantial amount of evidence to suggest that targeted PSMA therapy of PCa could be a particularly promising approach.
It is anticipated that this approach will lead to the development of new drug options for patients with advanced CRPC.
There are three primary categories of targeted PSMA immunotherapy: Antibody-drug conjugates (ADCs), chimeric antigen receptor T cells (CAR-T) and bispecific T cell redirection therapy. Each of these categories is outlined below.
Antibody-drug conjugates (ADCs)
The choice of antigens is a central factor in ADCs’ drug development process. It is important that these exhibit low or no expression in healthy cells but that these do express on the surface of tumor cells to promote the internalization of ADC transport into cells.
PSMA meets these requirements, and there has been good progress in the development of ADCs targeting PSMA.
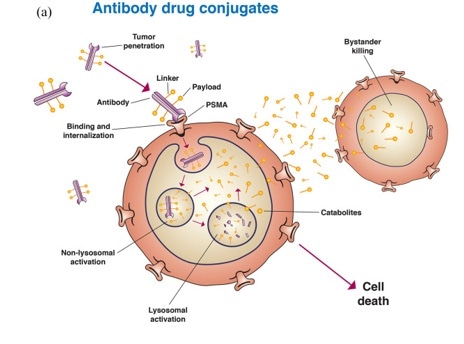
PSMA targeting in metastatic castration-resistant prostate cancer: where are we and where are we going? Doi: 10.1177/17588359211053898. Image Credit: ACROBiosystems
On August 3rd, 2021, Ambrx Announced that ARX517 – a PSMA-targeting antibody-conjugated drug (ADC) had completed its first patient-dosed Phase 1 clinical trial.
This specific multi-center, open-label, dose-escalation Phase 1 clinical trial had enrolled a total of 76 patients with advanced solid tumors who had previously failed standard therapy.
The team evaluated the safety, pharmacokinetics (PK) and antineoplastic activity of ARX517 in patients with PSMA-expressing tumors. These tumors were present in the treatment of prostate, lung, pancreatic and ovarian cancers.
On September 30th, Hengrui Pharmaceutical acquired a new drug patent for PSMA-targeting ADCs.
There is evidence to suggest that ADC’s therapeutic capacity in targeting PSMA in CRPC may be impacted by treatment-related adverse events, however. For example, MlN2704 is an ADC that conjugates a humanized J591 antibody to the Maytansine analog drug maytansinoid-1 (DM1).
A recent study of 62 patients treated with various regimens saw five patients had a PSA50 response and adverse events, including peripheral neuropathy.
Image Credit: ACROBiosystems
Chimeric antigen receptor T cells (CAR-T)
Chimeric antigen receptor T-cell (CAR-T) therapy is an emerging field that shows considerable promise.
CAR-T therapy involves acquiring a patient’s T cells and engineering these to enable them to express a T cell receptor directed against a specific antigen. These cells are then expanded before being reinfused back into the patient.
On August 31st, Poseida Therapeutics shared the news that P-PSMA-101 - its autologous CAR-T cell therapy - had provided positive preliminary results in Phase 1 clinical trial looking to treat patients with metastatic castration-resistant prostate cancer (mCRPC).
The therapy demonstrated encouraging activity in the first nine patients receiving low-dose treatment. P-PSMA-101 is a CAR-T cell candidate designed to target PSMA by genetically engineering the patient’s own T cells and empowering these to effectively and safely eliminate tumor cells.
A Phase I trial of CAR-T therapy designed to target PSMA featured five patients who received PSMA-targeted CAR-T cells and sustained infusions of low-dose IL-2 following pre-treatment chemotherapy.
It was observed that two of these five patients achieved the PSA50 response, while no patients were observed with CRS. This indicated that the use of PSMA-specific CAR-T cells plus IL-2 provided a more prominent antitumor response than monotherapy.
A further trial of seven patients with prostate cancer involved patients receiving PSMA-targeted CAR-T therapy following pre-treatment chemotherapy. CAR-T cells were found to persist in the patients’ blood for up to 2 weeks. One patient was even stable for more than 16 months.
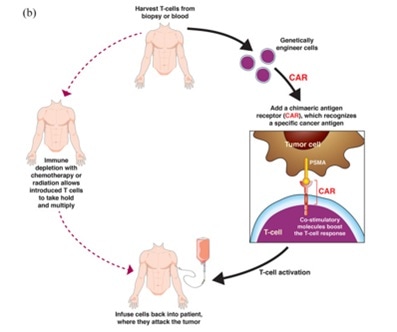
PSMA targeting in metastatic castration-resistant prostate cancer: where are we and where are we going? Doi: 10.1177/17588359211053898. Image Credit: ACROBiosystems
CAR-T therapy is highly effective in blood tumors, however, but its response rates are reduced in solid tumors. PSMA, therefore, would appear to be a highly suitable target for CAR-T cell therapy, but potential barriers remain, including the issue of immunosuppressive TME.
Image Credit: ACROBiosystems
Bispecific antibody therapy
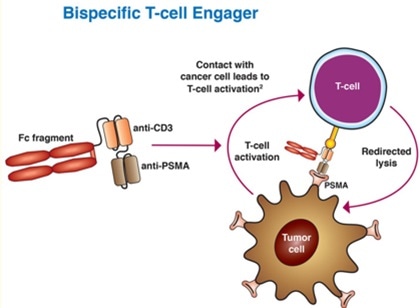
Bispecific T-cell engagers (BiTEs) represent an evolving field in prostate cancer treatment. BiTEs are bispecific monoclonal antibodies. One of these antibodies binds to T-cells via the CD3 receptor, while the other is targeted against a tumor-specific marker.
For example, AMG 212 is a dual antibody that targets CD3 and PSMA. A recent study involved 16 patients with mCRPC that had been divided into five different dose groups.
At the time of the data report, PSA levels in three patients had been found to decrease by more than half. One patient had been treated for 19.4 months and was exhibiting a complete regression of soft tissue metastases and a notable regression of bone metastases.
AMG 212 - like other bispecific drugs that target CD3 - has a short half-life, meaning that continuous intravenous infusion is required.
Amgen and BeiGene have since collaborated on the development of AMG 160, a new drug designed for the treatment of metastatic castration-resistant prostate cancer (mCRPC) in adults.
AMG 160 is new HLE-BiTE immunotherapy that possesses a longer half-life, allowing it to be administered at longer dosing intervals.
Preliminary results from the AMG 160 Phase 1 study indicated that 34% of patients responded to PSA50 with adverse effects, including CRS. There remains the possibility that mitigation strategies employed throughout the study could lower the rate of adverse reactions.
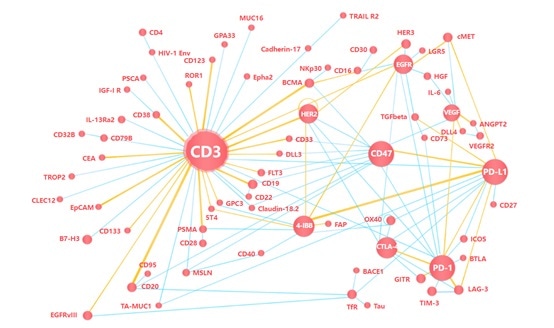
Image Credit: ACROBiosystems
PSMA targeting in metastatic castration-resistant prostate cancer: where are we and where are we going? Doi: 10.1177/17588359211053898. Image Credit: ACROBiosystems
PSMA has garnered considerable attention as a tumor marker that is specific to the surface of prostate cell membranes. This research has led to the development of bispecific antibody, RLT and CAR-T therapies designed to target PSMA.
A range of vaccine therapies, checkpoint blockade therapies, and other novel therapies, such as BiTEs, ADCs and CAR-T cells, present a series of fascinating and promising pathways towards the treatment of advanced prostate cancer.
Key products
ACROBiosystems has been at the center of the recombinant protein field for many years. The company has developed a series of high-quality PSMA proteins designed to accelerate PSMA-related research.
Various species of proteins are available, including human, mouse, rat and cynomolgus. Various tags are also available, including Fc, His, Fc and Avi, with protocols suitable for various applications, such as antibody screening and cell-based assays.
SDS-PAGE is also available with verification purity >95%, while SEC-MALS can be provided with verification purity >90%. High bioactivity has been verified by ELISA/SPR/FACS.
Product list
Source: ACROBiosystems
| Cat. No. |
Species |
Product Description |
Preorder/Order |
| PSA-HP2Q3 |
Human |
PE-Labeled Human PSMA / FOLH1 Protein, His Tag (Site-specific conjugation) |
Order |
| PSA-R5245 |
Rat |
Rat PSMA / FOLH1 Protein, His Tag (active enzyme, MALS verified) |
Order |
| PSA-C5247 |
Cynomolgus |
Cynomolgus PSMA / FOLH1 Protein, His Tag (MALS verified) (active enzyme) |
Order |
Click here to learn more about the product list.
Verification data
High purity verified by MALS
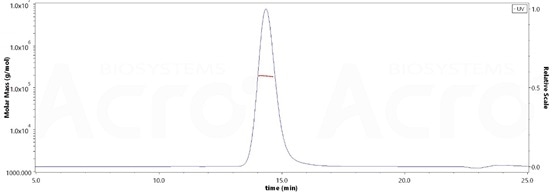
The purity of Rat PSMA, His Tag (Cat. No. PSA-R5245) is more than 90% and the molecular weight of this protein is around 170-208 kDa verified by SEC-MALS. Image Credit: ACROBiosystems
High bioactivity verified by ELISA
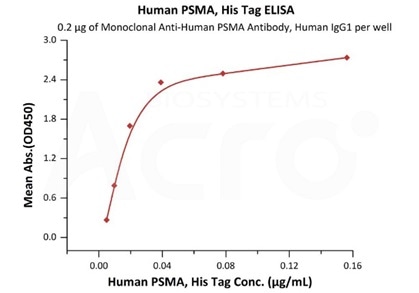
Immobilized Monoclonal Anti-Human PSMA Antibody, Human IgG1 at 2 μg/mL (100 μL/well) can bind Human PSMA, His Tag (Cat. No. PSA-H52H3) with a linear range of 2-39 ng/mL (QC tested). Image Credit: ACROBiosystems
High bioactivity verified by FACS
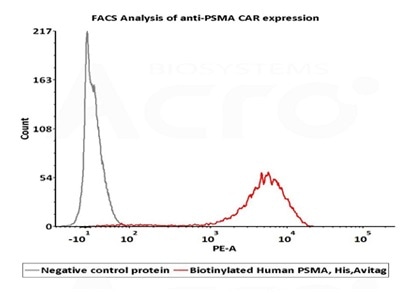
2e5 of PSMA-CAR-293 cells transfected with anti-PSMA-scFv were stained with 100μl of 1μg/mL of Biotinylated Human PSMA, His, Avitag (Cat. No. PSA-H82Qb) and negative control protein respectively, washed and then followed by PE-SA and analyzed with FACS (Routiney tested). Image Credit: ACROBiosystems
References
- Fay, E. K., & Graff, J. N. (2020). Immunotherapy in Prostate Cancer. Cancers, 12(7), 1752. https://doi.org/10.3390/cancers12071752
- Wang, F., Li, Z., Feng, X. et al. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis (2021). https://doi.org/10.1038/s41391-021-00394-5
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.