
Image Credit: ACROBiosystems
Epidemiology of Brain Tumors
Brain tumors are responsible for around 1.35% of all cancers and 29.5% of cancer-related fatalities. Brain tumors are classed as primary or metastatic based on their origin. Around 70% of brain tumors are benign, while the rest are malignant.
Glioblastoma, which arises from glial cells, is the most frequent malignant brain tumor. Glioblastoma has a five-year survival rate of fewer than 5%, with an average survival rate of one year.
A primary brain tumor is diagnosed at an average age of 60 years old, with a 75.2% survival rate. The average five-year survival rate for patients with benign brain tumors is 91.7%, whereas the relative five-year survival rate for patients with malignant brain tumors is 36% following diagnosis.
Furthermore, one-third of individuals with initial malignancies are thought to acquire metastatic CNS tumors during their illness.
As per Neurology, a Lancet sub-journal, China holds the primary position in terms of brain tumor incidence and death, with an annual incidence rate of 7/100,000, accounting for 2% of all tumors. Furthermore, because the skull encloses the brain, there is limited room for the tumor to expand.
As a result, the tumor will crush the nerves, blood vessels, and tissues around it. It has an impact on brain function, resulting in weakness, walking difficulties, loss of balance, partial or total blindness, difficulty comprehending or using language, memory issues, and so on.
Brain tumors, whether malignant or not, have a greater long-term and severe impact on patients’ quality of life than other forms of tumors.
Therapeutic Strategies for Brain Tumors
Chemotherapy, radiation therapy, and surgery are all popular treatments for brain tumors. Meanwhile, immunotherapy, which encourages a patient’s immune system to kill tumor cells, has been extensively researched as a therapeutic option.
Small molecule medicines, vaccines, monoclonal antibodies, bispecific antibodies, fusion proteins, ADC, and CAR-T are now being developed for brain malignancies, particularly glioblastoma. They target EGFR, VEGF, c-kit (CD117), TOP, PDGF, CSF-1, FGF, mTOR, PI3K, and Fit3.
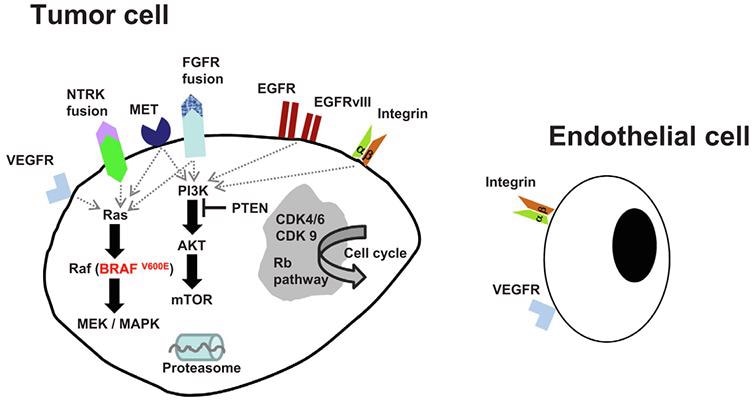
Candidate molecular pathways targeting interventions in glioblastoma. Image from reference
EphA2, for example, is highly expressed in glioblastoma but not in normal brain tissue, making CAR-T cell therapy targeting EphA2 an appealing option for improving therapeutic results. The traditional and possible therapeutic techniques for brain tumors, as well as new therapies, are discussed.
Classical therapeutic strategy: Inhibition of brain tumor growth
The role of tyrosine kinase in cancer and development is well understood. Tyrosine kinases make up more than half of proto-oncogenes and oncogene products, and their abnormal expression causes cell proliferation regulation to be disrupted, resulting in cancer.
Tumor invasion, metastasis, tumor neovascularization, and tumor chemoresistance are all linked to abnormal tyrosine expression. Antitumor drug development is now targeting more than 20 distinct families of receptor and non-receptor tyrosine kinases, including EGFR, VEGFR, PDGFR, FGFR, and others.
The anti-EGFR drug Nimotuzumab, for example, is frequently utilized in brain malignancies. When administered in children with high-grade gliomas, however, it has modestly increased overall survival.
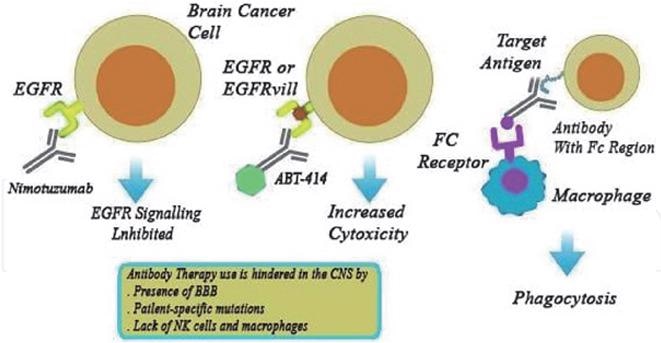
Mechanism of anti-EGFR monoclonal antibody in the treatment of brain tumors. Image from reference
The VEGF/VEGFR signaling pathway promotes tumor development and neovascularization. As a result, targeting this signaling system in anticancer drug development is an important approach to suppressing tumor neovascularization. Bevacizumab, a monoclonal antibody that targets VEGF, has recently been authorized in many countries for the treatment of glioblastoma.
Since monoclonal antibodies are unable to cross the blood–brain barrier, monoclonal antibody therapy in the brain has inferior survival benefits (BBB). One of the limitations is that they cannot break significant barriers. Antibody binding effectiveness is also affected by patient-specific antigen alterations.
Potential therapeutic strategy: Targeting intracellular signaling
The PI3K-AKT-mTOR system, which includes phosphatidylinositol 3-kinase (PI3K), protein kinase B (PKB/Akt), and target of rapamycin protein (mTOR), is an intracellular signaling mechanism that regulates cell cycle progression.
The Ras-MAPK pathway, which includes the serine protein kinase Ras and the mitogen-activated protein kinase (MAPK) tertiary cascade kinase, is crucial for signaling from the extracellular environment to the cell nucleus.
The STAT family of factors, as well as these pathways, their downstream signal transduction and transcriptional activity, are all linked to cancer and progression.
The positive expression rate of PI3K/AKT/mTOR in glioblastoma is strongly linked to the clinical grade and prognosis of the illness. As a result, the PI3K-AKT-mTOR signaling pathway has emerged as a possible therapeutic option for brain cancers.
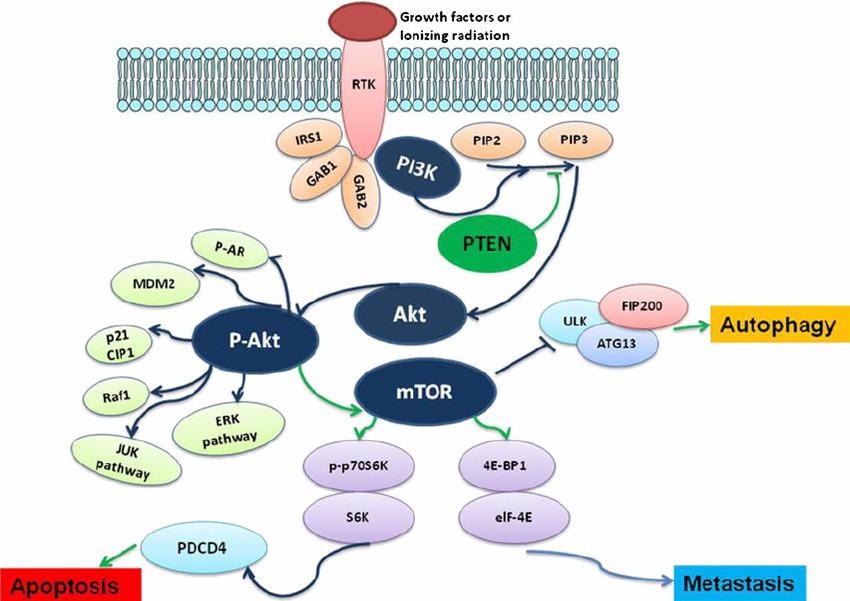
The role of PI3K-AKT-mTOR signaling pathway in the regulation of tumor metastasis, apoptosis, and autophagy. Image from reference
A variety of small-molecule compounds that target the PI3K-AKT-mTOR signaling pathway are now in phase I clinical trials.
These inhibitors include wortmannin, LY294002 and IC484068, which target PI3catalytic K’s subunit p110; fisetin (deguelin), a natural PI3K inhibitor; straurosporine, UCN-01, and perifosine, inhibitors of PDK, a serine/threonine kinase required for Akt activation; and rapamycin and its analogs RAD001, CI779, and AP23573, which are inhibitors that specifically target mTOR.
Rapamycin and its variants, which address downstream mTOR molecules selectively, have exhibited greater therapeutic outcomes in clinical studies and have promising prospects.
Innovative therapeutic strategy: Anti-brain tumor vaccines
Since brain tumor cells have particular proteins on their surfaces, vaccinations that target these proteins can allow the immune system to precisely kill tumor cells without damaging healthy cells.
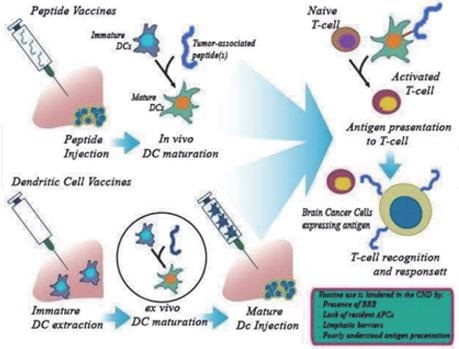
TPolypeptide vaccines and dendritic virus vaccines for the treatment of brain tumors. Image from reference
SurVaxM, the world’s first peptide-mimetic tumor vaccine, has shown safety and tolerability in patients with malignant glioma in phase I research. It is composed of natural amino acids that decompose when an immune response is triggered, with minimal side effects.
SurVaxM vaccine has a favorable safety profile, good tolerability, and the capacity to prolong survival even in the most difficult-to-treat patient subgroup, according to the latest data from the phase II research. The FDA designated SurVaxM vaccine as an orphan medicine in 2017.
AV-GBM-1 is a dendritic cell vaccine made from autologous cells. The vaccine contains antigenic information taken from post-operative tumor tissue, which will be sent to T cells to drive tumor-killing activity once administered.
On April 8th, 2020, phase II clinical data for AV-GBM-1 were released, demonstrating that this innovative vaccination has the potential to significantly increase general survival in patients with newly diagnosed glioblastoma.
The results of phase II clinical trial reported at the ASCO conference in June 2021 demonstrated that this vaccination has the potential to enhance overall survival and progression-free survival in patients with newly diagnosed glioblastoma when compared to traditional treatment regimens.
IGV-001 is an autologous tumor cell vaccine that targets tumor cells in numerous ways by utilizing the patient’s immune system as a defensive mechanism. To begin, it employs glioblastoma patients’ surgically removed tumor cells.
These cells are then treated with IGF-1R antisense oligonucleotides (IMV-001) to induce controlled cell death in the tumor cells. The cells are then combined with an excess of IMV-001 (as an adjuvant) in an infiltration chamber before being transplanted into the patient’s belly.
These cells must be transplanted within 24 hours following surgery, and they must be withdrawn 48 hours later. The FDA has also designated this revolutionary vaccination as an orphan medication.
Early detection and treatment of brain tumors are critical for preventing lasting brain damage and death. ACROBiosystems utilizes an application-oriented development strategy, with a focus on product design, quality control, and solution-based support, as a major manufacturer of recombinant proteins and other critical reagents for assistance in developing target therapeutics, vaccines, and diagnostics.
Aneuro is a new ACRO product line that focuses on and reflects devoted neuroscience research activities. By supplying high-quality protein products and useful new ideas, brain research can be further nurtured and stimulated.
References
- Le Rhune, et al. (2019) Molecular targeted therapy of glioblastoma. Cancer Treat Rev, doi:10.1016/j.ctrv.2019.101896
- Pichaivel, M., et al. (2022). An Overview of Brain Tumor. IntechOpen. doi:10.5772/intechopen.100806
- Chang, L. et al. (2014) Emerging roles of radioresistance in prostate cancer metastasis and radiation therapy. Cancer Metastasis Rev. doi:10.1007/s10555-014-9493-5
- Platten, M, et al. (2021) A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature.
- https://medicalxpress.com/news/2021-03-first-ever-vaccine-malignant-brain-tumors.html
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Our mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. We supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, we deliver solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. We empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.