Johnson & Johnson and Xencor signed a cooperation agreement on October 4th, 2021, where Johnson & Johnson paid $100 million in advance and made a $25 million equity investment in Xencor.
Additionally, it was required to pay a milestone amount of $1.188 billion to collaboratively create and commercialize plamotamab and novel XmAb® B-cell that target bispecific antibodies designed to conditionally activate T cells through the CD28 co-stimulatory receptor.
In September 2021, the Center for Drug Evaluation (CDE) of China proclaimed that Roche’s CD3/CD20 bispecific antibody RO7030816 (Mosunetuzumab) received clinical trial implied permission for the treatment of relapsed/refractory follicular lymphoma.
Review the development process of antibody drugs from CD3 and CD20
Targeting CD20 and CD3 is believed to have observed the complete mechanism in antibody drug development. At the start of the mid-19th century, “bactericidin” was observed in the serum of patients infected with specific diseases and that they could treat related diseases. This was the first understanding of antibodies and took over a century to put forth the model from “bactericidin” to antibody.
The development of real therapeutic antibody drugs dates back to the 1970s. Milstein and Kohler fused antibody-producing B cells with tumor cells to create hybridoma cells that can produce antibodies and multiply indefinitely. This technology was a landmark in the creation of therapeutic antibody drugs. Milstein and Kohler won the Nobel Prize in Physiology or Medicine in 1984 for their work.
The first therapeutic antibody-drug Orthoclone OKT3 was approved by the US FDA during the second year of the Nobel Prize for hybridoma technology. This antibody targets CD3 and prevents immune rejection after kidney transplantation.
Orthoclone OKT3 is of pronounced significance as the first approved therapeutic antibody drug. Yet, as its sequence is of mice origin, it gets eliminated by the human immune system as an exogenous protein during administration. This results in a significant decrease in the therapeutic impact after numerous administrations, and might, at times, produce serious immune-related side effects and even death.
These adverse effects cast a shadow over the production of therapeutic antibody drugs.
Based on this, the murine variable region was chimerized with the human constant region to create a chimeric antibody. This antibody could identify the target protein and decrease the proportion of murine sequence, which at the start decreases the heterologous rejection generated by the sequence.
The representative drug among the chimeric antibodies is the Rituxan — the monoclonal antibody targeting CD20 approved by Roche in 1997 for treating B-cell lymphoma. The progression of antibody-drug research and development led to the development of various humanized antibodies generated through sequence modification or optimization.
Fully human antibodies generated with transgenic animals or phage/yeast display technology slowly matured, facilitating the development of antibody drugs appearing an explosive leap.
Antibody drug development targeting CD20
CD20 (membrane-spanning 4-domains A1, MS4A1), a four-pass transmembrane phosphoprotein, plays a role in controlling the activation and proliferation of B cells. The differentiation of B lymphocytes from pluripotent stem cells occurs in the bone marrow and develops through progenitor B cells, pre-B cells, immature B cells and mature B cells.
Mature B cells are discharged into lymphoid tissues and they continue to differentiate into plasma cells. CD20 is a surface marker of B cells. Its expression starts later and is lost earlier when compared to CD19, during B cell development and differentiation. CD20 is expressed from pre-B cells to mature B cells, but not on pro-B cells and plasmablasts.
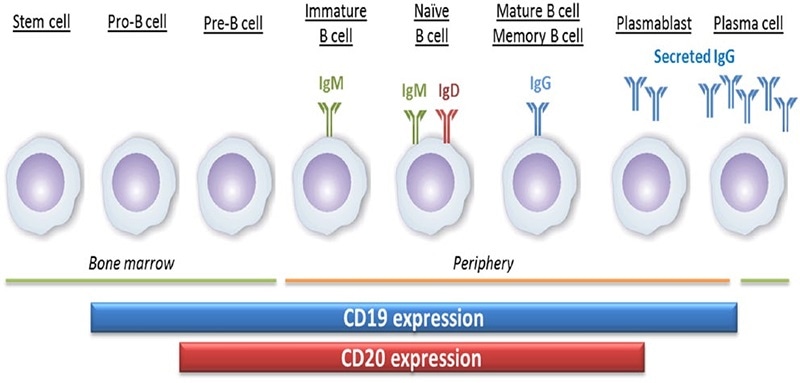
The expression of CD20 and CD19 during the development of B cells. Image Credit: ACROBiosystems
CD20’s expression on B cell-derived leukemia, lymphoma and other tumor cells, and B cells involved in inflammatory and immune-related diseases makes it a therapeutic target for leukemia, lymphoma and autoimmune diseases.
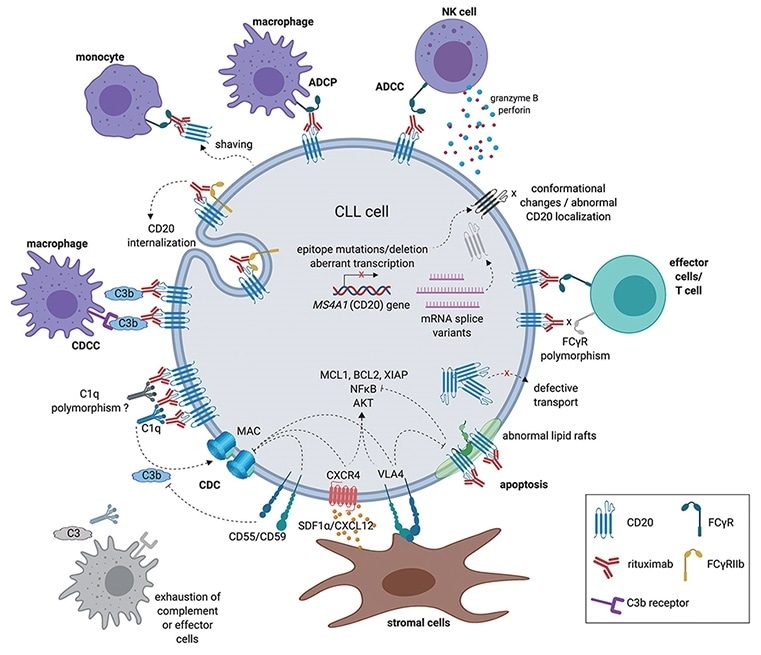
The mechanism of action of anti-CD20 antibodies. Image Credit: ACROBiosystems
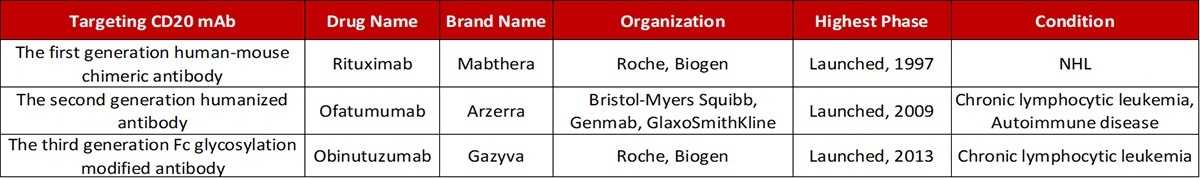
Antibody drugs targeting CD20 have undergone three generations of innovation. The first generation is Rituximab — a chimeric IgG1 mAb that attaches to the B lymphocyte surface antigen CD20. It is widely considered that Rituximab depletes B lymphocytes through ADCC and complement-dependent cytotoxicity (CDC) mechanisms.
The second generation is Ofatumumab — a completely humanized IgG1 anti-CD20 mAb. Even though the immunogenicity and side effects are decreased, the antibody specificity and the affinity of the antigen-binding had a specific decline.
The third generation is Obinutuzumab, where the Fc region was glycosylated to enhance binding capacity to effector cells — including natural killer cells, neutrophils, thus potentially improving ADCC. Yet, clinical data reveals that even though the anti-CD20 monoclonal antibody has advanced to the third generation, the leading position of Rituximab was not shaken.
Progress in the development of mAbs targeting CD20. Source: ACROBiosystems
CD20 can recruit T cells to B cells by attaching to CD3 and other targets for the generation of bispecific antibodies, which achieves the destroying effect of T cells on B cells.
Epcoritamab (GEN3013) produced by Genmab is recent in the field of CD20/CD3 bispecific antibody creation.
It is a subcutaneously injected bispecific antibody and is currently being researched for the treatment of B-cell non-Hodgkin's lymphoma. Roche has created two bispecific antibodies with various structural forms that target CD20/CD3 — Mosunetuzumab and Glofitamab — simultaneously.
At the ASH annual conference in 2020, Roche revealed the results of the drug's clinical trials. The overall efficiency rate attained 86% in patients who were given Mosunetuzumab combined with CHOP in the treatment of relapsed/refractory non-Hodgkin’s lymphoma, and 71% of patients achieved complete remission.
The recent clinical research of Mosunetuzumab cover multiple indications like diffuse large B-cell lymphoma, follicular lymphoma and non-Hodgkin’s lymphoma. Glofitamab is a bispecific antibody containing a special structure created by Roche, which has two domains that can attach to CD20 and one that attaches to CD3.
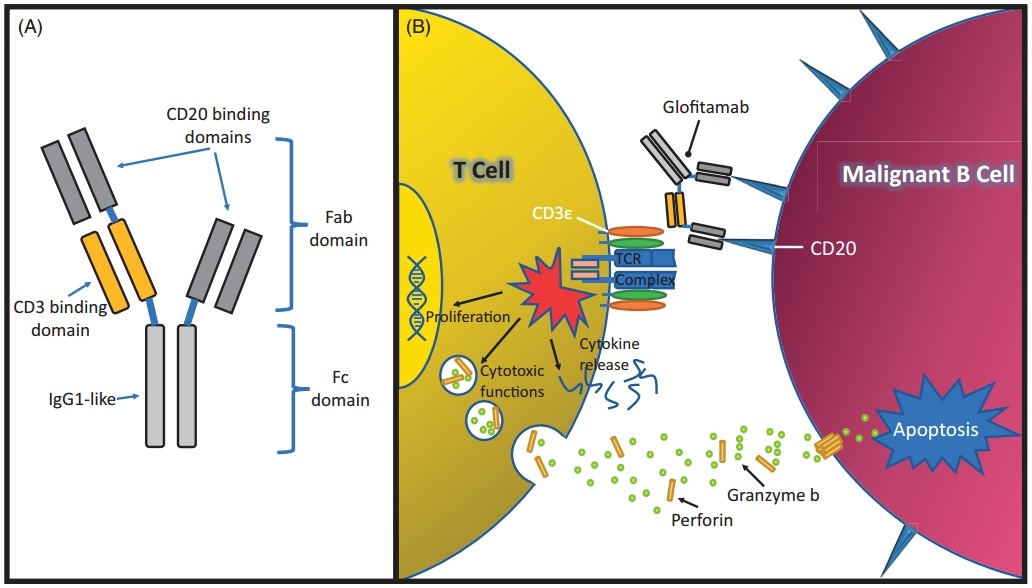
The structure and mechanism of Glofitamab. Image Credit: ACROBiosystems
At the ASH annual conference in 2019, Roche proclaimed the clinical trial progress data of Glofitamab combined with the third-generation CD20 mAb (Otuzumab) for the treatment of relapsed/refractory non-Hodgkin’s lymphoma. The objective response rate (ORR) of combined treatment was 54% (n = 15/28), and the complete response rate (CR) was 46% (n = 13/28).
Competitive landscape: from CD20 mAbs to CD20/CD3 bispecific antibodies
The bigger market opened up by the CD20 monoclonal antibody made pharmaceutical companies worldwide join the track. To avoid competition and achieve product differentiation, pharmaceutical companies rely on the research and development of bispecific antibodies, which have an upsurge in CD20/CD3 bispecific antibody drug development.
ACROBiosystems offers full-length CD20 and CD3 series target proteins. To promote developing drugs targeting CD20, ACROBiosystems specifically uses multiple technology platforms to create full-length four-pass transmembrane target protein CD20 expressed by HEK293.
Full-length CD20 proteins. Image Credit: ACROBiosystems
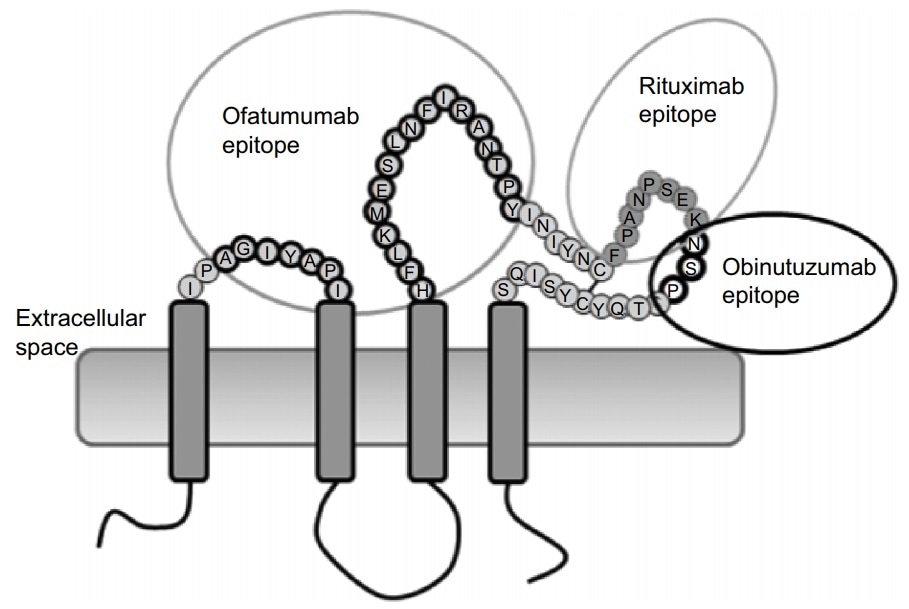
- Native conformation and complete epitope: The identification epitope of Rituximab and Obinutuzumab is a large loop of the extracellular domain of CD20, and Ofatumumab attaches to both large loop and small loop
- Highly bioactive: The biological activity is verified by attaching Rituximab and Ofatumumab, showing high affinity at the nanomolar level, all protocols are offered for free
- Larger product catalog: CD20-Nanodisc, CD20-VLP, CD20-DDM/CHS, including unconjugated and biotinylated protein
- Suitable for ELISA/SPR/BLI/ELISA/SPR/BLI/FACS
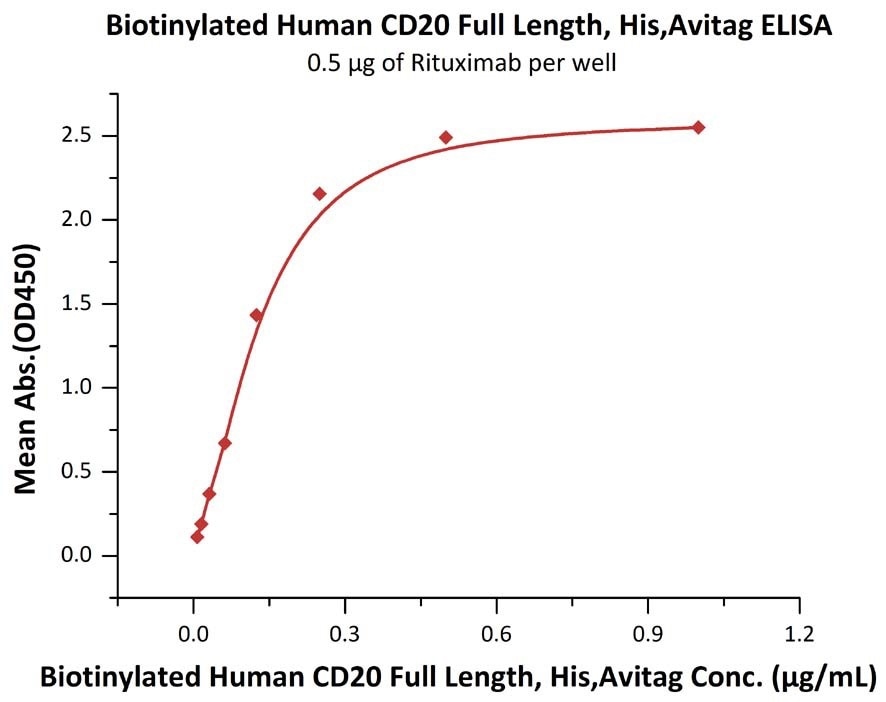
Immobilized RTX at 5 μg/mL (100 μL/well) can bind Biotinylated Human CD20 Full Length, His, Avitag (Cat. No. CD0-H82E3) with a linear range of 0.008-0.125 μg/mL. Image Credit: ACROBiosystems
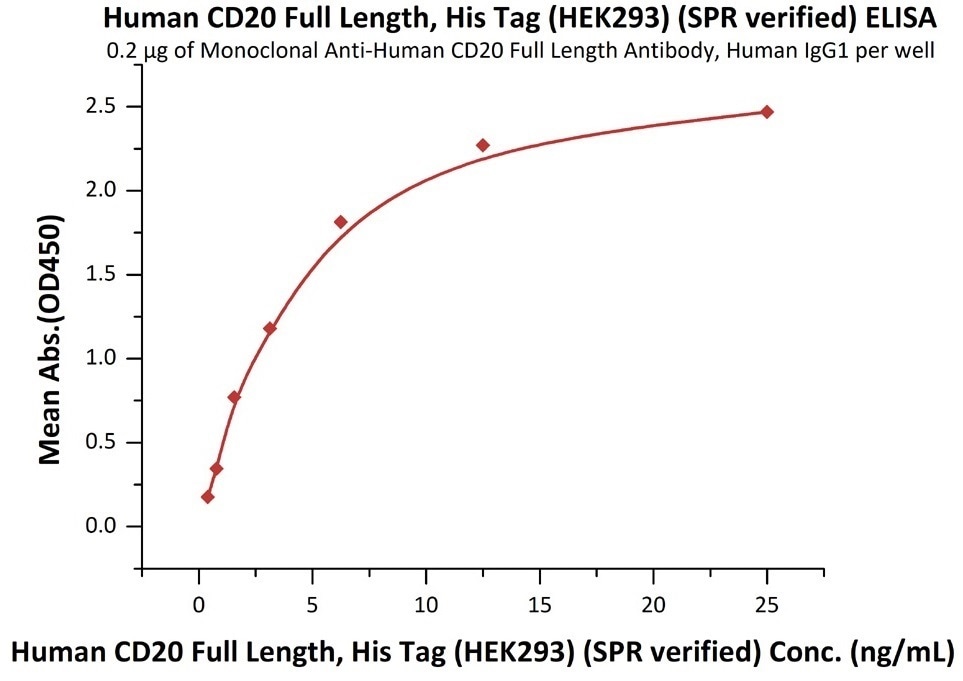
Immobilized anti-CD20 antibody at 2 μg/mL (100 μL/well) can bind Human CD20 Full Length, His Tag, HEK293 (SPR verified) (Cat. No. CD0-H52H3) with a linear range of 0.4-6 ng/mL (in presence of DDM and CHS). Image Credit: ACROBiosystems
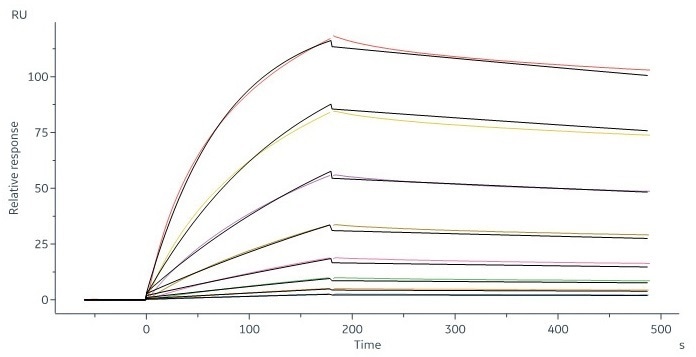
Human CD20 Full Length, His Tag, HEK293 (SPR verified) (Cat. No. CD0-H52H3) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind RTX with an affinity constant of 6.21 nM as determined in an SPR assay (in presence of DDM and CHS) (Biacore 8K). Image Credit: ACROBiosystems
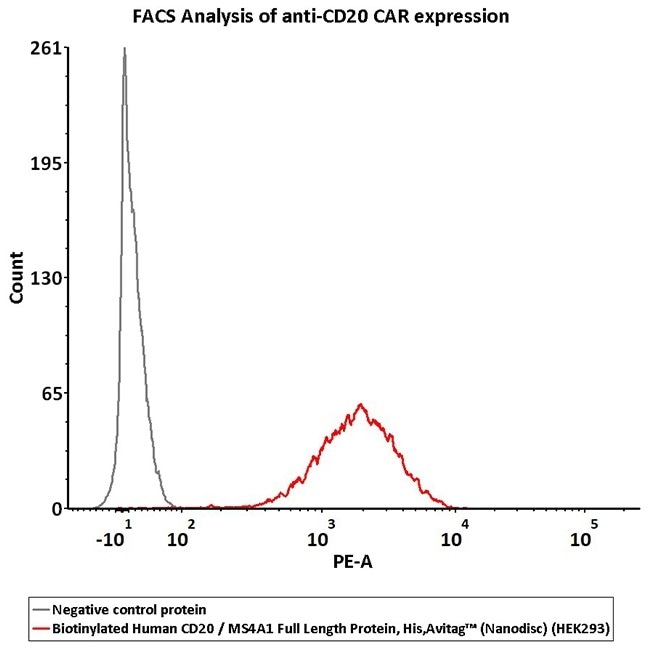
2e5 of CD20-CAR-293 cells transfected with anti-CD20-scFv were stained with 100 μL of 3 μg/mL of Biotinylated Human CD20 Full Length, His, Avitag (Cat. No. CD0-H82E3) and negative control protein respectively, washed and then followed by PE-SA and analyzed with FACS (QC tested). Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.