The tumor microenvironment (TME) constitutes the tumor, extracellular matrix (ECM) and several non-transformed cells, such as immune infiltrates, fibroblasts and vascular vessels recruited from adjacent local or distant tissues.
TME has a major role in tumor initiation, progression, invasion, metastasis and resistance by deploying growth factors, matrices, vascular networks and cytokines for nutrient and waste exchange. Understanding TME necessitates a close investigation of signaling pathways responsible for its characteristics.
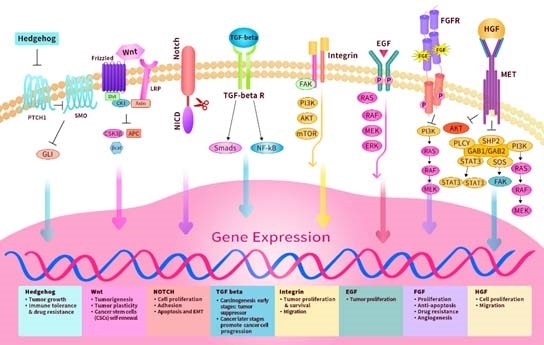
Image Credit: ACROBiosystems
The Hedgehog, Notch, Wnt, etc. are classes of evolutionarily highly conserved signaling pathways that govern cellular fate. These pathways are upregulated in TME, such as cancer stem cells, and act as vital targets in cancer treatment.
Hedgehog pathway
The canonical Hedgehog (HH) pathway includes the autocatalysis of HHC, secretion of HHN ligand and the addition of cholesterol molecule to the N-terminus. When the HHN binds to its cognate receptor Patched (PTCH1), its inhibitory effect is released on Smoothened (SMO), leading to the downstream discharge of GLI transcription factors.
The HH pathway has a major role not only in embryonic development but also in adult tissue regeneration and maintenance. Hyperactive HH signaling — overexpression of HH or mutation in PTCH and SMO — leads to basal cell carcinoma and lung, pancreatic, breast, prostate and ovarian cancer.
Upregulated HH signaling in tumor cells in TME boosts the proliferation and survival of adjacent tumor cells through autocrine signaling. HH ligand synthesized by epithelial cells can signal mesenchymal/stromal cells, which signal back to epithelial cells, thus boosting the growth and survival in a paracrine signaling way.
Notch pathway
The Notch signaling pathway — a juxtacrine (contact-dependent) signaling pathway — mediates cell fate decisions between adjacent cells and plays a role in each component of the TME and in the interaction between various parts of the TME. The Notch receptor, which is a transcription factor secured at the membrane, is released after interaction with a cognate ligand.
When the Notch ligand binds, the Notch regulatory region (NRR) unfolds and unmasks the cleavage sites for ADAM10/17 and the γ-secretase. Cleavage at NPR results in the release of the Notch intracellular domain (NICD) into the cytosol. The Notch ligands govern the NICD production dynamic and strength.
These ligands are transmembrane proteins of the Delta/Serrate/LAG-2 (DSL) class. In the case of mammals, this class includes two jagged ligands (Jag1 and Jag2) and three delta-like ligands (Dll1, Dll3 and Dll4).
Different compartments of the TME widely express Notch receptors and ligands. Notch signaling has been identified to take part in the activation of fibroblasts, angiogenesis and maintenance of the cancer stem cell niche. It also shapes the immune infiltrate at the tumor site and may be controlled by physical and chemical heterogeneity in the TME.
Wnt
The Wnt signaling pathway is a vital regulator of tissue homeostasis, wound repair, embryogenesis development, stemness control and malignancy. Dysregulated Wnt signaling has a crucial role in the initiation, progression and metastasis of different types of human cancers.
There are normally three pathways: the non-canonical Wnt/planar cell polarity (PCP) pathway, the canonical Wnt pathway and the non-canonical Wnt-calcium pathway.
In the case of the canonical Wnt pathway, the Wnt ligand attaches to the Frizzled (FZD) receptor — a G protein-coupled receptor (GPCR) including seven transmembrane domains and the co-receptor low density lipoprotein receptor-related protein 5/6(LRP5/6).
Thus, β-catenin accumulates and translocates to the nucleus, and there, it can bind to the transcription factor T-cell factor/lymphoid enhancer factor (TCF/LEF) and engage the transcriptional Kat3 co-activator p300 and/or CREB-binding protein (CBP) to stimulate downstream Wnt target genes.
Wnt-activated genes play major roles in various processes in oncogenesis and development, like proliferation, self-renewal, metastasis and differentiation. By contrast, non-canonical Wnt signaling governs the stabilization of proteins besides β-catenin to preserve intracellular functions through these alternative pathways.
TGF-β pathway
Transforming growth factor-beta (TGF-β) is a pleiotropic growth factor. The TGF-β signaling, a conserved pathway, arises from multicellular organisms and has a crucial role in various cellular and developmental processes. TGF-β binds to a single TβRII receptor, bringing together two TβRI and two TβRII receptors to form a hetero-tetrameric complex.
Such a ligand-mediated assembly activates the phosphorylation and stimulation of TβRI by TβRII-initiation of downstream signaling pathways with their intracellular serine/threonine kinase domains. Either canonical (SMAD-dependent) or non-canonical (SMAD-independent) signaling pathways transduce the signal.
TGF-β signaling pathway is highly crucial in TME that has dual roles in carcinogenesis. In the early stages, TGF-β serves as a tumor suppressor that inhibits tumor growth by suppressing cell cycle progression in cancer cells and triggering apoptosis in pre-malignant cells.
Yet, tumor-inhibiting effects of TGF-β often vanish in advanced stages of tumors as its tumor pro-invasive functions prevail. The consequently higher TGFβ secretion in the tumor microenvironment influences cancer cell growth and triggers migration, invasion and angiogenesis.
Moreover, it is proposed that TGF-β might play a crucial role in molding the CAF landscape, specifically because it is associated with myofibroblast differentiation and plays a role in tumors and in other fibrotic diseases.
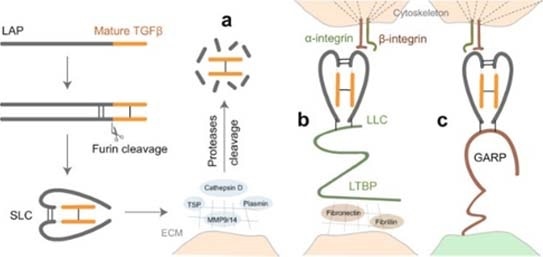
Image Credit: ACROBiosystems
The latent TGF-β signaling is triggered first with cleavage of its proprotein into the latency-related peptide (LAP) and the mature TGF-β. Then, the LAP dimer binds to mature TGF-β to form a small latent complex (SLC). This latent TGF-β is activated by three key mechanisms:
- Proteases that occur in the extracellular matrix (ECM) cleavage of LAP and the release of active TGF-β; on the other hand, thrombospondin (TSP) can also lead to the activation of TGF-β by direct binding to LAP.
- The SLC can be secured to the ECM proteins through latent TGF-β-binding protein (LTBP) and develops what is called the large latent complex (LLC), which will discharge active TGF-β by cell contraction following interaction between integrins and LAP.
- SLC that binds to glycoprotein A repetition predominant protein (GARP) on the cell surface interacts with integrin to release active TGF-β. Different cancer therapeutics that target TGF-β with antibodies, small molecules, ligand traps and antisense oligos are being investigated in clinical trials.
Integrins
Integrins are a class of cell adhesion receptors governing the adhesion of cells to ECM and include α and β heterodimeric subunits with 24 different heterodimers identified so far. Integrins also have a crucial role in cell signaling and control the growth, survival, migration and invasion of cells.
Moreover, Integrin signaling is associated with other receptor tyrosine kinase (RTK) signaling and a mediator of downstream signal transduction involving MAPK/Erk, PI3-Akt, JNK and SAPKs pathways. In the case of TME, Integrin promotes the binding of tumor cells to ECM, angiogenesis and has a key role in cancer cell metastasis and aggressiveness.
Thus, researchers are targeting Integrins for anticancer therapy using integrin blockers. Two candidates — an Arg-Gly-Asp (RGD) pentapeptide that targets avb3 and avb5 integrins and volociximab as well as IgG4 inhibitor of a5b1 — are in phase II and III clinical trials.
RTKs
Receptor Tyrosine Kinases (RTKs) are a family of cell surface transmembrane receptors forming cross-linked dimers through ligand binding, resulting in the activation of its tyrosine kinase activity through cross-phosphorylation and then signal transduction. RTKs adheres to cytokines, growth factors and hormones to induce a range of key cellular processes and also have major roles in TME.
EGF
Nearly all non-neoplastic cell types express endothelial growth factor receptor (EGFR), besides the mature cells of the lymph hemopoietic system. Specifically, in the non-neoplastic cells of the TME, active EGFR signaling is hypothesized to play a supportive role in the proliferation, angiogenesis and metastasis of tumor cells.
EGFR activation is induced by ligand binding and receptor dimerization and then cross phosphorylation through Src homology 2 (SH2) and phosphotyrosine binding (PTB) domains. The downstream signaling pathways triggered by EGFR are the PI3K pathway, KRAS-BRAF-MEK-ERK pathway, as well as AKT and STAT pathways among others.
In the case of TME, EGFR signaling activates the production and secretion of several angiogenic regulating factors, like Interleukin-8 (IL-8), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF). Moreover, tumor growth factor-alpha (TGF-α) and EGF act as an effective pro-angiogenic factor that controls tumor-induced angiogenesis.
The main Ras/PI3K pathway downstream signaling following EGFR activation results in the abnormal expression of VEGF in the TME. A range of therapeutics target the EGF pathway and EGF-stimulated production of VEGF, such as kinase inhibitors gefitinib, monoclonal antibody cetuximab and PD15035.
FGF
Fibroblast growth factor receptors (FGFRs) are RTKs that contribute to different biological processes such as the regulation of tissue development and repair. Variations in FGFRs 1–4 — like amplification, fusions and mutations, as well as abnormal transcriptional or epigenetic regulation and variations in tumor-stromal interactions in the tumor microenvironment — can result in the development and/or advancement of cancer.
When carcinogenesis occurs, FGF activates the autophosphorylation of FGFR at a main tyrosine residue in an activation loop of the tyrosine kinase domain. FGFR autophosphorylation leads to a change in the structure of the tyrosine kinase domain from an inactive to an active form.
Then, the transduction of FGF signals to the RAS-MAPK occurs, and branching of PI3K-AKT signaling through FGFRs and FRS2 takes place. Moreover, FGF signals are transduced to the DAG-PKC, and branching of IP3-Ca2+-releasing signaling occurs through FGFRs and cell growth factor PLCγ.
FGF signals play a role in the proliferation, stemness, drug resistance, anti-apoptosis, epithelial-to-mesenchymal transition (EMT), angiogenesis and invasion in target cells.
HGF
In general, the hepatocyte growth factor (HGF), also called scatter factor, is secreted by stromal cells. It is the native peptide ligand of HGFR/c-MET receptor.
The abnormal stimulation of HGFR/cMET has a crucial role in the development and advancement of various human cancer types, such as breast, lung, thyroid, renal and gastrointestinal carcinomas, as well as sarcomas and malignancies of the nervous system, like glioblastoma multiforme (GBM) as well as others.
The HGF/MET axis induces the activation of various downstream signaling pathways like PI3K/Akt/mTOR, RAS/RAF/MEK/ERK, as well as JAK/STAT and Wnt/β-catenin. HGF/MET controls several biological processes in cancer cells like cell survival, proliferation, apoptosis suppression, invasion, migration, drug resistance and metastasis.
Summary
The TME is a complex setting, including a range of signaling molecules, soluble factors, chemokine and ECM that promotes the proliferation, survival and invasion of cancer cells. This setting is promoted by stromal cells surrounding the tumor made of epithelial cell boosting tumor angiogenesis, immune cells, fibroblast-discharging chemokines and inflammatory cells.
This network between the tumor and stroma cells is crucial. Moreover, gaining insights into and targeting these signaling pathways will allow the development of potent cancer therapeutics against solid tumors that exhibit historically poor prognoses.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.