For customers who want to do quantification of human protein biomarkers through Mass Spectrometry (MS) analysis, ACROBiosystems now offer a range of heavy-labeled proteins alongside a material generation service.
The proteins are expressed through 293 cells in a chemically defined environment where the protein will be labeled with [U- 13C6, 15N4]-L-Arginine and [U- 13C6, 15N2]-L-Lysine. These proteins will be excellent standards for both identification and quantification standards, due to the correct post-translational modifications mechanism of human cell.
Product Highlights
Heavy-labeled Protein Products
- An incorporation efficiency higher than 99.9%
- Authentic posttranslational modification and protein conformation
- Greater accuracy and consistency than synthetic peptide
- Compatible with all types of MS equipment
- Custom protein generation service available
- Guaranteed quality and short lead-time
Heavy-labeled Protein Services
- Guaranteed to have greater than 99.9% incorporation efficiency
- 4-6 weeks lead time from sequence to end product
- Host cell has been adapted to serum-free, chemically defined SILAC medium over 10 passages
- Serum contained medium avoids residual amino acid interface in traditional approach
| Comparison of ACROBiosystems protein based MS Standard Vs. Other Company peptide based MS standard |
| |
ACRO protein |
Other Company peptide |
| Reproducibility |
High |
Low |
| Accuracy |
High |
low |
| Precision |
High |
low |
| Identification of best SRM and MRM transitions |
Yes |
No |
| PT modification and processing |
Yes |
No |
| Coverage |
>10,000s |
100s |
Case Study
Case 1: rh ADAM12 MS Standard
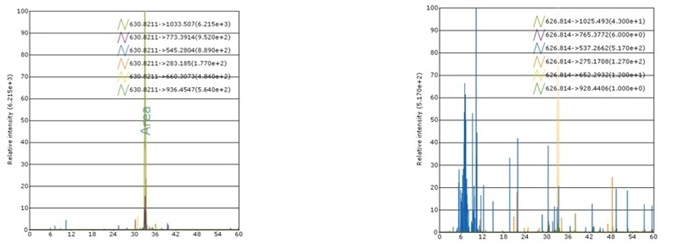
Recombinant Human ADAM12 MS Standard Protein, C13 and N15-labeled (rhADAM12, Heavy Labeled) Arg 29 - Asp 513 (Accession # AAH60804.1) was produced through human 293 cells (HEK293) in chemically defined medium.
Case 2: rh Apo-A1 MS Standard
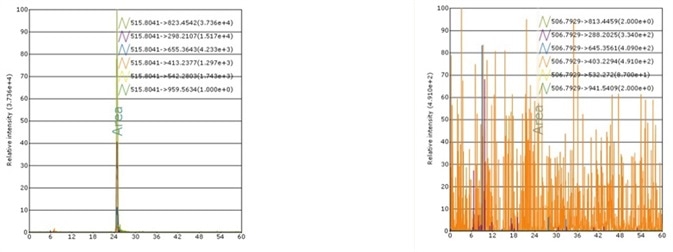
Recombinant Human Apo-A1 MS Standard Protein, C13 and N15-labeled (rh Apo-A1, Heavy Labeled) Asp 25 - Gln 267 (Accession # NP_000030.1) was produced through human 293 cells (HEK293) in chemically defined medium.
Heavy-labeled Protein Products
Stable isotope labeling by amino acids in cell culture (SILAC) is a popular method for (MS)-based quantitative proteomics. Based on mass spectrometry, the technique determines variation in protein abundance among samples using non-radioactive isotopic labeling.
SILAC uses normal metabolic processes to label cellular proteomes, incorporating non-radioactive, stable isotope containing amino acids into the proteins. The method uses the incorporation of amino acids with substituted stable isotopic nuclei (e.g. 13C, 15N) as the basis for measurement.
For example, the growth medium can contain arginine labeled with six carbon-13 atoms (13C), heavy amino acid, as opposed to the normal carbon-12 (12C), light amino acids. After five cell doublings, cells grown in this medium have incorporated the heavy amino acids. SILAC amino acids do not affect cell morphology or growth rates, they incorporate the heavy amino acid, 13C6-arginine, into all cell proteins (Figure 1).
SILAC has emerged as a standout tool in the study of cell signaling, post translation modifications such as phosphorylation, protein–protein interaction and regulation of gene expression. On top of this, SILAC has become a crucial method in the global study of secreted proteins and secretory pathways, known as secretomics.
It can be used to distinguish between proteins secreted by cells in culture and serum contaminants. There are also published works on standardized protocols of SILAC for various applications.
The process works by differentially labeling cells by growing them in light medium with normal arginine (Arg-0, blue color) or medium with heavy arginine (Arg-6, red color). When the proteins begin metabolic incorporation of the amino acids there is a mass shift of the corresponding peptides.
A mass spectrometer then detects this mass shift, indicated by the depicted mass spectra. When both samples are combined, the ratio of peak intensities in the mass spectrum indicates the relative protein abundance. In this example, the labeled protein has the same abundance in both samples (ratio 1).
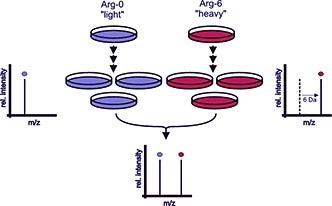
Figure 1. The principle of SILAC
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.