Pathologists first observed several cases of an abnormal increase in vascularization of human tumors more than a century ago. Ide et al. were the first to postulate the existence of vascular growth-stimulating factors produced by tumor cells to support oncogenesis in relation to these phenomena.

Image Credit: ACROBiosystems
Dr. Judah Folkman first suggested the new notion of preventing tumor angiogenesis as a possible method of treating cancer and other malignancies in 1971. However, it was not until 1983 that vascular endothelial growth factor (VEGF), a factor that increased vascular permeability and was relevant to tumor angiogenesis, was identified.
VEGF is an important regulator of angiogenesis in embryonic development, skeletal growth, and reproductive function and has been implicated in pathological angiogenesis associated with tumors, intraocular neovascular diseases, and other diseases.
Despite being discovered in 1983, the Pharma Projects database still places VEGF among the top five after Her-2, EGFR, CD3E, and CD19.
Due to the crucial role that VEGF plays in controlling both physiological and pathological angiogenesis, it has become an “inspiring” target for both existing and novel drugsover the past two decades.
VEGF family: Ligands and receptors
Understanding the diverse functions of VEGF in physiologic processes is essential for comprehending the implications and potential of VEGF as a therapeutic target. The placental growth factor (PIGF) with three tyrosine kinase receptors, VEGFR-1, VEGFR-2, and VEGFR-3, and five primary subtypes, VEGF-A, VEGF-B, VEGF-C, and VEGF-D, make up the complicated VEGF signaling system.
While all subtypes have some degree of homology to VEGF, the original VEGF refers primarily to VEGF-A, which is essential for angiogenesis and maintenance. There are several isoforms of VEGF-A, including VEGF121, VEGF165, VEGF189, and VEGF206. Each isoform has unique molecular characteristics, with VEGF165 being the most prevalent and has the best bioavailability and biopotency.
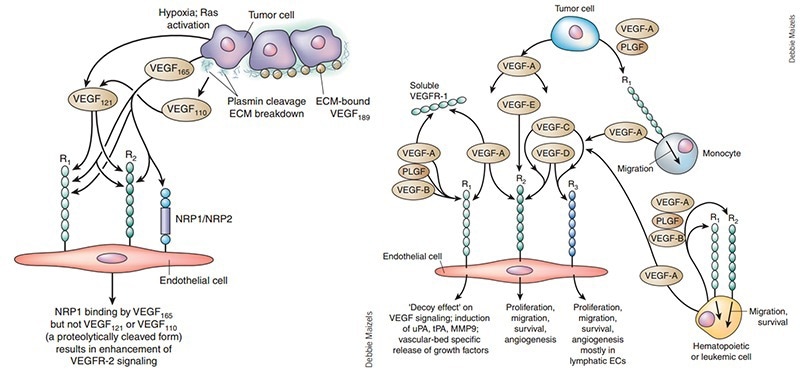
Interaction of VEGF with receptors. Image Credit: ACROBiosystems
There are variations among VEGF receptors in terms of their selectivity and affinities to various ligands. The majority of biological responses to VEGF are known to be mediated by VEGFR-2, which also plays a significant role in angiogenesis, microvascular permeability, and endothelial cell mitosis and survival.
The role of VEGF in tumors
The physiological mechanisms of angiogenesis and microvascular permeability affect the development of blood vessels from pre-existing capillaries. These crucial mechanisms for embryonic development and adult wound healing are mediated by VEGF and its receptors. However, this signaling system is altered to promote the growth of tumors.
VEGF is increased during oncogenesis by oncogene expression, a number of growth factors, and hypoxia. The growth factor VEGF, together with other growth factors released by the tumor, activates the angiogenic switch, causing new blood vessels to develop around the tumor and promoting exponential growth.
The resulting blood vessels are bent, uneven in shape, and lack venules, arterioles, and capillaries because of the aberrant rise in angiogenesis. Additionally, these veins bleed and leak, which raises interstitial pressure close to the tumor. Due to the inadequate blood flow caused by these abnormal arteries, hypoxia is exacerbated, which leads to increased VEGF synthesis and, eventually, angiogenesis.
Representative drugs targeting VEGF in progress
VEGF is a potent target for anticancer therapy due to the crucial role it plays in tumor angiogenesis. Monoclonal and bispecific antibodies (bsAb) that target VEGF are currently the focus of a growing number of businesses. Three typical VEGF-targeting drugs are highlighted below.
A humanized anti-VEGF IgG1 monoclonal antibody called bevacizumab was created by Roche and is the first anti-angiogenic drug approved for treatment in a range of tumor types. By directly attaching to VEGF, the antibody blocks tumor-initiated angiogenesis, which in turn stops the spread and development of the tumor.
For the treatment of metastatic colorectal cancer, bevacizumab was licensed by the FDA in 2004, recognized in Europe in 2005, and introduced in China in 2010. The drug was authorized in 2015 to treat non-small cell lung cancer.
Currently, non-small cell lung cancer, colorectal cancer, renal cell carcinoma, glioblastoma, cervical cancer, fallopian tube cancer, ovarian cancer, peritoneal cancer, and many other types of cancer are among the approved indications of use.
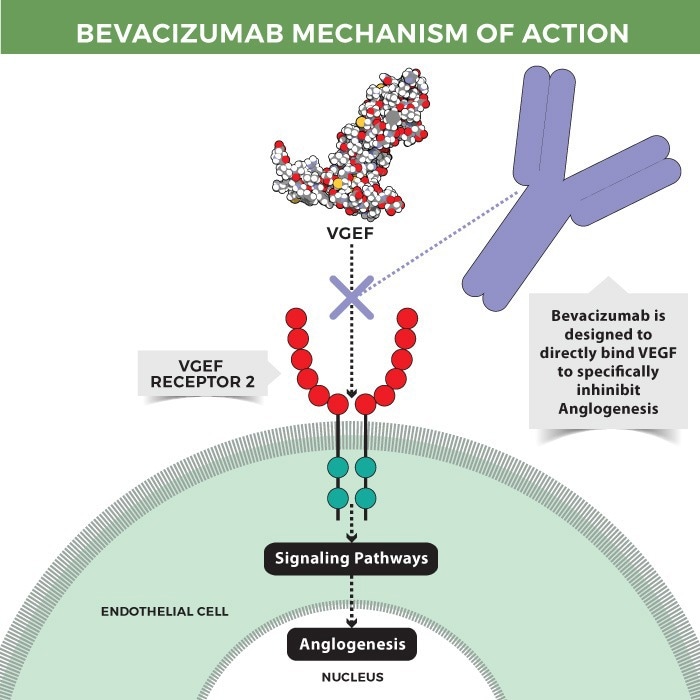
Mechanism of action of bevacizumab. Image Credit: ACROBiosystems
Akeso’s PD-1/VEGF bsAb (AK112), which is based on the company’s distinctive TETRABODY technology, is one of the well-known drugs. This drug is now being tested in patients as a PD-1 antibody and VEGF blocker combination therapy for a variety of tumor types, such as non-small cell lung cancer, renal cell carcinoma, and hepatocellular carcinoma.
Since AK112 simultaneously blocks two targets, it can function more efficiently than a single agent, resulting in increased antitumor activity.
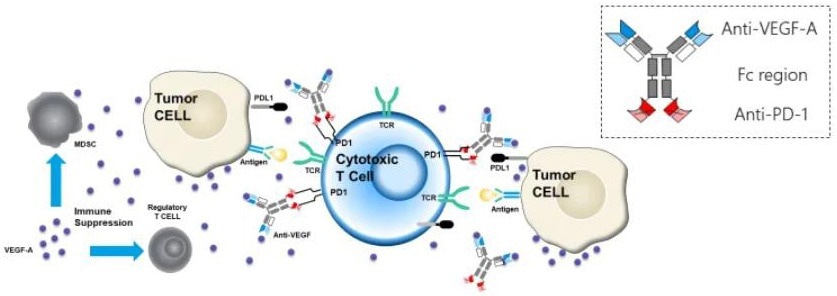
Mechanism of action of AK112. Image Credit: ACROBiosystems
Roche also has a bsAB drug named Faricimab that targets Ang-2 and VEGF-A for use in ophthalmic diseases. There is currently little information available, but Roche has claimed that this drug may be used to treat diabetic macular edema and neovascular age-related macular degeneration (nAMD) (DME).
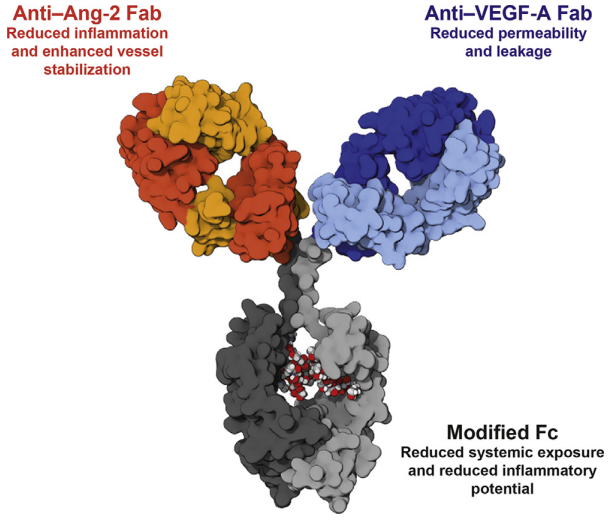
Structure of Faricimab. Image Credit: ACROBiosystems
To sum up, VEGF has been implicated in a variety of human cancers as an underlying facilitator for tumorigenesis and disease development. A treatment plan that targets VEGF/VEGFR offers a special entry point into the tumor’s vascular microenvironment.
As a result, VEGF possesses a variety of traits that make it a desirable target for prospective therapies. The vascular microenvironment surrounding the tumor serves as the entry point for the targeted therapeutic method that targets VEGF/VEGFR.
The permeability of tumor drugs can also enhance their anti-tumor impact. On one hand, it can cut off the nutritional source of tumor cells and prevent tumor growth; On the other hand, the anti-tumor effect can be enhanced by the permeability of tumor drugs.
To aid in the development of VEGF/VEGFR antibody medicines, ACROBiosystems has introduced a range of HEK293-expressed VEGF family proteins with high purity, multi-species, multi-tags, and highly bioactive VEGF family proteins.
Product list
Source: ACROBiosystems
| Molecule |
Cat. No. |
Species |
Product Description |
| VEGF165 |
VE5-H4210 |
Human |
ActiveMax® Human VEGF165 Protein, Tag Free (MALS verified)HOT |
| VE5-H5248 |
Human VEGF165 Protein, His TagHOT |
| VE5-H8210 |
Biotinylated Human VEGF165, epitope tag free, ultra sensitivity (primary amine labeling)HOT |
| VE5-H82Q0 |
Biotinylated Human VEGF165 Protein, His, Avitag™HOT |
| VE5-HF248 |
FITC-Labeled Human VEGF165 Protein, His TagHOT |
| VEGF121 |
VE1-H4213 |
ActiveMax® Human VEGF121 Protein, Tag FreeHOT |
| VE1-H5246 |
Human VEGF121 Protein, His Tag (MALS verified)HOT |
| VE1-H82E7 |
Biotinylated Human VEGF121 Protein, Avitag™, His TagHOT |
| VEGF110 |
VE0-H5212 |
ActiveMax® Human VEGF110 Protein, Tag Free (HPLC-verified) |
| VEGF120 |
VE0-M4211 |
Mouse |
ActiveMax® Mouse VEGF120 Protein, Tag Free |
| VE0-M82Q2 |
Biotinylated Mouse VEGF120 Protein, His, Avitag™ |
| VEGF164 |
VE4-M4216 |
ActiveMax® Mouse VEGF164 Protein, Tag Free (HPLC-verified) |
| VE4-M82Q3 |
Biotinylated Mouse VEGF164 Protein, His, Avitag™ |
| VEGF-C |
VEC-H4225 |
Human |
Human VEGF-C / Flt4-L Protein, His TagHOT |
| VEGF-D |
VED-H5228 |
Human |
Human VEGF-D Protein, His Tag |
| VEGF R1 |
VE1-H5220 |
Human |
Human VEGF R1 / Flt-1 Protein, His Tag |
| VE1-H82E3 |
Human |
Biotinylated Human VEGF R1 / Flt-1 Protein, His, Avitag™ |
| VE1-M5256 |
Mouse |
Mouse VEGF R1 / Flt-1 Protein, Mouse IgG2a Fc Tag, low endotoxin |
| VE1-R5257 |
Rhesus macaque |
Rhesus macaque VEGF R1 / Flt-1 Protein, Mouse IgG2a Fc Tag, low endotoxin |
| VEGF R2 |
KDR-H5227 |
Human |
Human VEGF R2 / KDR Protein, His Tag (MALS verified) |
| KDR-H5280 |
Human VEGF R2 / KDR Protein, Strep Tag (MALS verified) |
| KDR-H82E5 |
Biotinylated Human VEGF R2 / KDR Protein, Avitag™, His Tag |
| VE2-C52H3 |
Rhesus macaque |
Rhesus macaque VEGF R2 / KDR Protein, His Tag |
| VE2-H5255 |
Human |
Human VEGF R2 / KDR Protein, Fc Tag |
| VE2-HF254 |
FITC-Labeled Human VEGF R2 / KDR Protein, His Tag |
| VE2-M5258 |
Mouse |
Mouse VEGF R2 / KDR Protein, Mouse IgG2a Fc Tag, low endotoxin |
| VEGF R3 |
FL4-H5251 |
Human |
Human VEGF R3 / FLT4 Protein, Fc Tag (MALS verified) |
| FL4-H82E1 |
Biotinylated Human VEGF R3 / FLT4 Protein, His, Avitag™ |
| FL4-M5251 |
Mouse |
Mouse VEGF R3 / FLT4 Protein, Mouse IgG2a Fc Tag, low endotoxin |
Product features and verification data
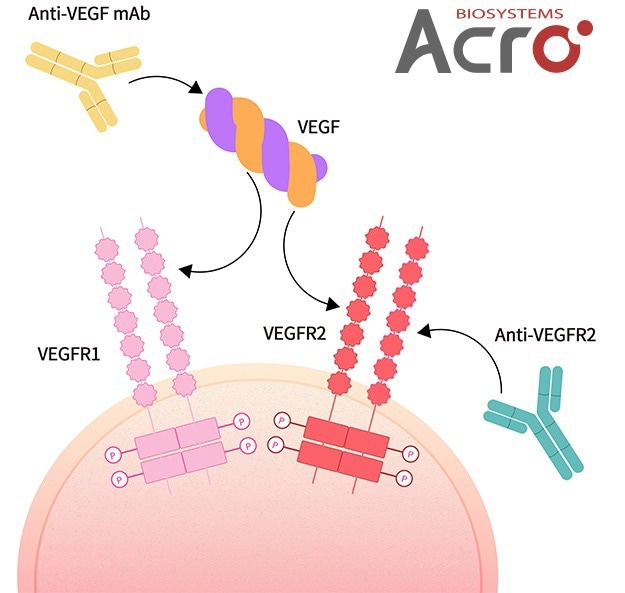
Image Credit: ACROBiosystems
High purity and homogeneous structure verified by SDS-PAGE and SEC-MALS
(Note: Native VEGF is a 45 kDa heparin-binding glycoprotein with a homodimer structure, the correct and homogeneous structure is significant to decrease the risk of drug development.)
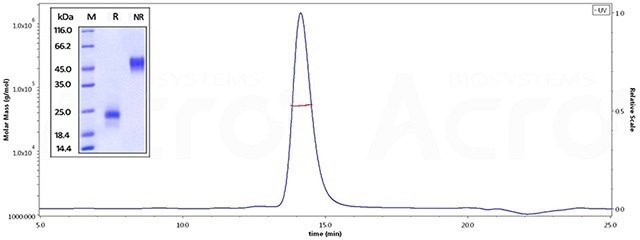
ActiveMax® Human VEGF165, Tag Free (MALS verified) (Cat. No. VE5-H4210) on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 98%. The purity of ActiveMax® Human VEGF165, Tag Free (MALS verified) (Cat. No. VE5-H4210) is more than 95% in HP-SEC, and the molecular weight of this protein is around 40–55 kDa verified by SEC-MALS. Image Credit: ACROBiosystems
High biological activity verified by ELISA
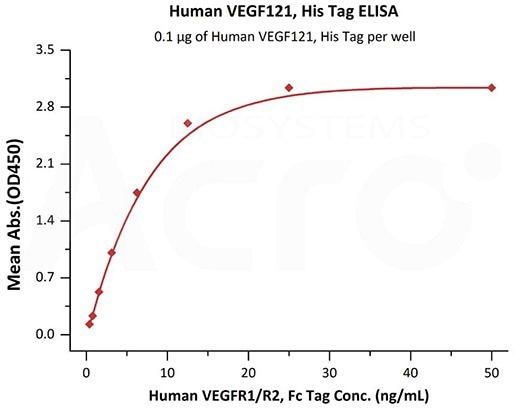
Immobilized Human VEGF121, His Tag (Cat. No.VE1-H5246) at 1 μg/mL (100 μL/well) can bind Human VEGFR1/R2, Fc Tag with a linear range of 0.4-6 ng/mL (QC tested). Image Credit: ACROBiosystems
High biological activity verified by SPR
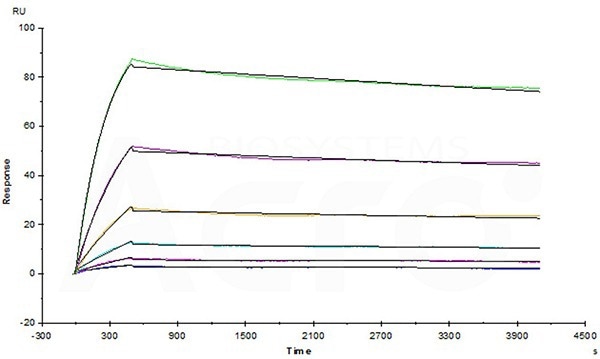
Avastin (Bevacizumab) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human VEGF165 Protein, His Tag (Cat. No. VE5-H5248) with an affinity constant of 1.03 nM as determined in a SPR assay (Biacore T200). Image Credit: ACROBiosystems
High biological activity verified by cell-based assay
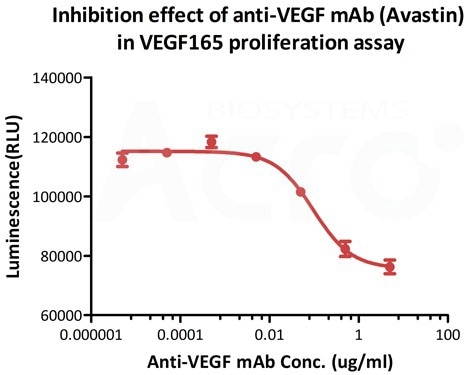
Inhibition assay shows that the proliferation effect of ActiveMax® Human VEGF165, Tag Free (MALS verified) (Cat. No. VE5-H4210) is inhibited by increasing concentration of anti-VEGF mAb (Avastin). The concentration of VEGF165 used is 20 ng/mL. The ED50 is 0.065-0.229 μg/Ml. Image Credit: ACROBiosystems

Image Credit: ACROBiosystems
References
- Napoleone Ferrara, Hans-Peter Gerber, et al. The biology of VEGF and its receptors. Nature Medicine. 2003, 9(6): 669–676.
- Peter Carmeliet. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005;69 (suppl 3):4–10.
- Jayashree Sahni, Sunil S. Patel, et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema. American Academy of Ophthalmology. 2019: 1155–1170.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.