An antibody-drug conjugate (ADC) is a newly developed, highly-targeted type of cancer therapeutic. ADCs are created by grafting monoclonal antibodies to a cytotoxic payload.
This new type of cancer therapy is a form of vectorized chemotherapy, which has huge potential to combat many types of cancer that have been difficult to treat effectively, improving the clinical prognosis for cancer patients.
The efficacy of ADCs can be affected by various factors during their development. These factors include the quality of monoclonal antibodies, the selected target protein, the stability of linkers, payload, and, most importantly, the conjugation method employed.
Random conjugation is typically used, leading to a lack of stability and variable efficacy of treatments. Therefore, site-specific conjugation methods are highly preferable as they precisely control the conjugation site. This produces a homogenous mixture, which can improve ADC design.
This article will look at antibody-drug conjugates and ACROBiosystems’ AGLink site-specific conjugation kit for clinical professionals in cancer therapy.
Antibody-drug conjugates
An antibody-drug conjugate is created using chemical linkers to attach monoclonal antibodies to a cytotoxic payload. During the development of an efficacious ADC, its key components and the development strategy must be considered carefully.
The final efficacy and safety of the antibody-drug conjugate are affected by these elements. Therefore, clinicians and researchers should know each component and strategy during drug design.1
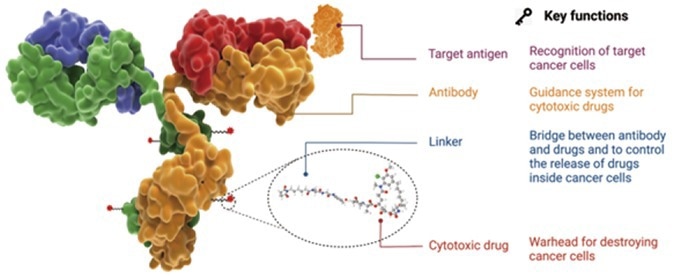
Figure 1. The structure, components and functions and characteristic of an ADC drug. 1 Image Credit: ACROBiosystems
The drug-to-antibody ratio (DAR) is a key characteristic of ADC therapeutics. This ratio elucidates the number of drug molecules bound to a single antibody. Depending on the conjugation strategy, the number of drug molecules bound to a single molecule can vary significantly.
The overall potency of an antibody-drug conjugate is reduced by low-drug loading, but if higher amounts of the drug are loaded, there is the risk of higher cytotoxicity, increased side effects, and altered pharmacokinetics.
Appropriate conjugation strategy selection affects ADC homogeneity and therapeutic effectiveness.2,3
Non-specific conjugation on pre-existing cysteine or lysine residues via coupling reactions can occur when conventional conjugation techniques are employed. Consequently, DAR can vary from zero to eight, producing heterogeneous ADCs. This can occur at various linker positions.1-3
Site-specific conjugation technologies
Various site-specific conjugation technologies have been developed recently to increase ADC efficacy. These methods include using unnatural amino acids, engineered cysteine residues, and glycosyltransferases to enhance enzymatic conjugation. These techniques can help obtain more homogenous ADCs.3
The clinical effectiveness of these methods has been demonstrated through improvements in antibody-drug conjugate therapeutic index and pharmacokinetics. Moreover, enhanced conjugation site control reduces linker-payload overall hydrophobicity. This avoids unintended payload release into the patient’s system.3
AGLink site-specific conjugation kits for ADC development
ACROBiosystems have developed the AGLink site-specific conjugation kit to improve the efficacy and safety of ADCs. This technology utilizes an enzymatic modification method (the one-pot process) to reduce antibody N-glycans and enable controllable site-specific conjugation.
Antibody N-glycans are reduced via fucosylation, which is the process of adding fructose sugar units to a target molecule. A recent study by the company evaluated the resulting ADCs after conjugation for homogeneity, cytotoxicity, and immunoreactivity.
The study
AGLink ADC site-specific conjugation kits (ACROBiosystems, Glyco-therapy Biotechnology; Cat. No. ADC-P001; Cat. No. ADC-P002 ) were used and based on the conjugation platform YTConjuTM (Glyco-therapy Biotechnology Co., Ltd.).4
Conjugation method
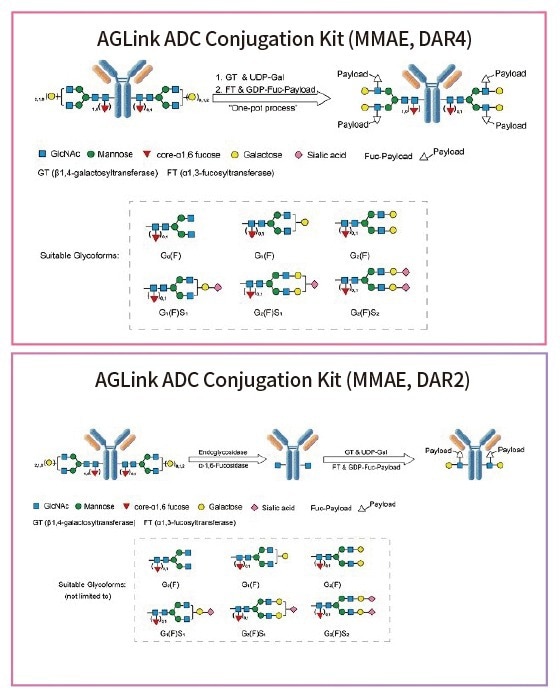
Figure 2. Conjugation mechanisms using the AGLink ADC Conjugation Kit. Two different conjugation methods are proposed above with either a DAR of 2 or 4. Suitable glycoforms that are compatible for enzymatic modification are listed below. Image Credit: ACROBiosystems
Study results
MMAE and Trastuzumab were used to characterize AGLink’s efficacy. The researchers identified N-glycans at the CH2 domain’s aspargine 297 positions on each Fc heavy chain fragment. Glycosylation reduced these N-glycans to form payload-linked reactive sites.
Results indicated that the resulting glycosylation produced varied amounts of mannose, galactose, N-acetylglucosamine, fucose, and sialic acid. These were in the form of various complex-type biantennary structures.
Resulting Trastumuzab-MMAE ADCs with DARs of two or four were developed and characterized.
High homogeneity conjugated antibodies
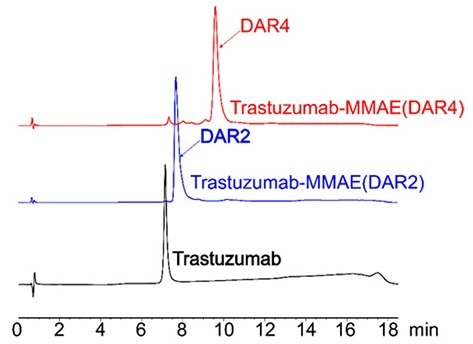
Figure 3. HIC-HPLC analysis of MMAE ADCs (DAR 4 & DAR 2). A shift in retention time was observed for higher DAR ADCs result associated with to the hydrophobic payload (MMAE). After conjugation using AGLink ADC kit, it can be observed that the resulting ADCs are highly homogenous for both DAR2 and DAR4 conjugation mechanisms. Image Credit: ACROBiosystems
Preserved bioactivity after conjugation
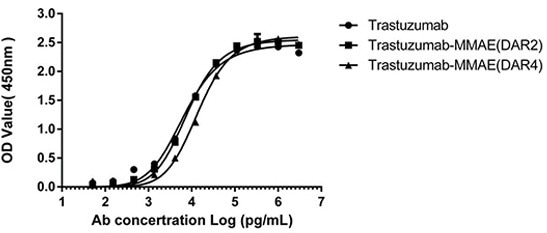
Figure 4. ELISA analysis was performed to test the antigen-binding capacity of both DAR2 and DAR4 ADCs. HER2 antigen binding to Trastuzumab-MMAE (DAR2/4) was unaffected after the AGLink conjugation, preserving antibody bioactivity. Image Credit: ACROBiosystems
Consistency in the site-specific labeling process mitigates aggregation
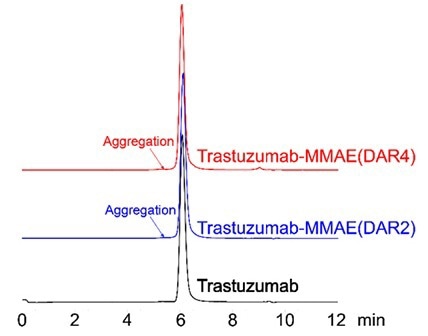
Figure 5. SEC-HPLC was performed to evaluate ADC aggregation. No observable peaks were seen in the resulting chromatogram, with less than 5% of antibody aggregation. Image Credit: ACROBiosystems
In vitro plasma stability test for AGLink ADCs
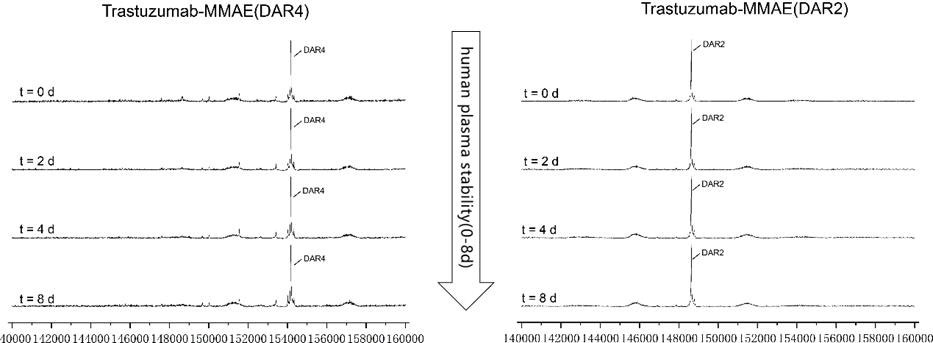
Figure 6. MMAE ADCs (DAR2/4) were spiked into human plasma and tested over 8 days and evaluated through LC-MS. Both types of ADCs were stable in vitro throughout the experiment. Image Credit: ACROBiosystems
In vitro cytotoxic activity assay
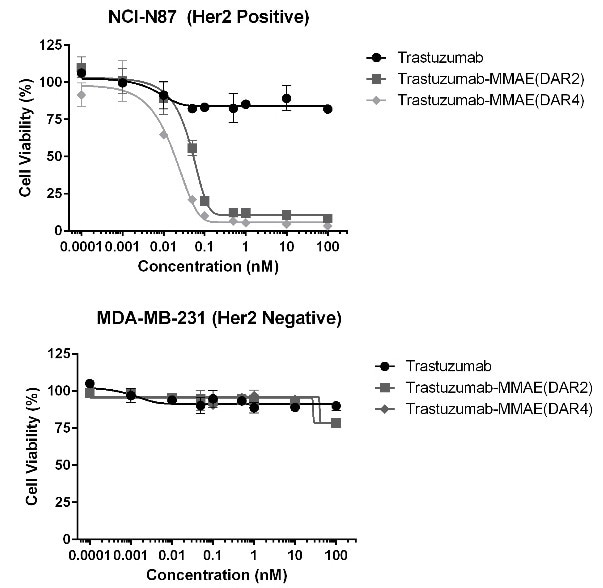
Figure 7. MMAE ADCs (DAR 2/4) were evaluated on its cytotoxicity to HER2 positive and negative cell lines. The ADCs developed in this study were proven to induce cell death in HER2 positive cell lines, while preserving cell viability in HER2 negative cells. Image Credit: ACROBiosystems
Conclusion
ADCs are a rapidly evolving class of novel cancer therapeutics. Due to the problems with conventional conjugation and preparation techniques, site-specific modifications are becoming more frequently employed to meet the demands of this field of research.
Amongst these new modification methods, glycoengineering has emerged as a suitable approach for site-specific conjugation methods. The AGLink site-specific conjugation kit from ACROBiosystems utilizes glycoengineering to produce ADCs with high degrees of homogeneity and efficacy.
Enzymatic modification of IgF Fc glycans via glycoengineering is used to perform conjugation in the AGLink kit. No prior amino acid sequence is required using this technology. Stable antibody conjugates with minimal batch variation are produced using ACROBiosystems innovative technology.
The system provides clinical and research scientists working in the field of cancer therapy with a powerful tool that offers powerful site-specific conjugation and can aid preclinical development of MMAE-ADCs. Contact the experts at ACROBiosystems to learn more about their innovative technologies.
References and Further Reading
- Zhiwen, F et al. (2022) Antibody drug conjugate: the "biological missile" for targeted cancer therapy.[J] .Signal Transduct Target Ther, 7: 93.
- Theocharopoulos, C et al. (2021) Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines (Basel). 9(10):1111.
- Marei Hany E, et al. (2022) Potential of antibody-drug conjugates (ADCs) for cancer therapy.[J] .Cancer Cell Int, 22: 255.
- Yang Y, et al. (2022) Reducing the Complexity of Fc Glycan Enables the Construction of Antibody Conjugates with Unexpected High Efficiency and Payload Capacity via Glycoengineering[J].
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.