Chimeric antigen receptor T cell therapies, also known as CAR-T therapies, are a significant breakthrough in treating hematological malignancies. These therapies include Yescarta and Kymriah.
There are, however, some critical limitations in currently approved CAR-T therapies. Their main limitation is their inability to effectively treat solid tumors. Overcoming this crucial hurdle is a primary research goal in cell therapy. This is especially critical as solid tumors account for over 90% of all diagnosed cancers.
Unlike their hematological counterparts, solid tumors comprise various tissue, immune, and tumor cells, creating a cellular milieu termed the tumor microenvironment. The tumor microenvironment is both a physical and chemical barrier, preventing the infiltration of external CAR cells and limiting cell therapy potential.
The tumor microenvironment is extremely complex, with abnormal stromal composition and vascular structure and a variety of trapped immunosuppressive cells which secrete and maintain immune suppressive signals, hindering the efficaciousness of cell therapy.
Alongside this, the diversity of tumor cells within the tumor microenvironment makes it extremely difficult for tumor-specific antigens to be properly selected. The specificity of CAR-T cells can be manipulated, causing additional challenges.
In recent years, scientists have developed strategies to overcome these significant barriers to effective cell therapy for cancer patients with solid tumors. Novel approaches include full-length transmembrane protein platforms. These advances offer off-the-shelf items with high purity and quality for analysis.
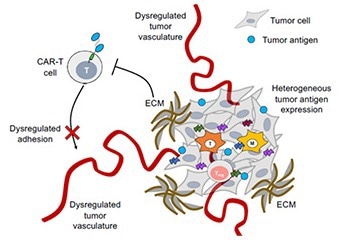
Figure 1. Poor penetration of CAR-T cells into the tumor microenvironment due to dysregulated tumor vasculature. 1 Image Credit: ACROBiosystems
Enhancement of CAR-T cell infiltration ability
Penetrating the tumor microenvironment to effectively target and treat a solid tumor is a key hurdle in cell therapy. This difficulty significantly decreases the efficacy of current techniques, leading to negative health outcomes for cancer patients.
Improved adhesion is a vital first step for improving the infiltration of the tumor parenchyma. One strategy that has been explored in CAR-T therapies to improve adhesion is targeting integrin avβ3, which is a cellular receptor that is highly expressed on the surface of neovascular cells on a solid tumor.
This method can significantly improve the elimination of avβ3-positive tumor cells. Within a murine xenograft model, this approach eliminated melanoma lesions in studies.
Other studies have utilized chemotactic techniques, which target chemokines secreted by tumor cells. These chemokines then bind to receptors on modified T cell surfaces, enhancing infiltration into the tumor microenvironment and targeting and treating solid tumors.
Reversing immune suppression within the tumor microenvironment
The tumor microenvironment fosters an immunosuppressive environment. In the tumor microenvironment, immunosuppressive cytokines and cells are promoted, while various immune activators are inhibited.
To combat this immunosuppressive environment, several researchers have promoted approaches based on the overexpression of inflammatory cytokines such as interleukin-12, 15, and 18.
CAR-T cells modified with a self-expressing cytokine gene that modulates and offsets the TME’s immunosuppressive environment are a research target. These are termed “armored” CAR-T cells.
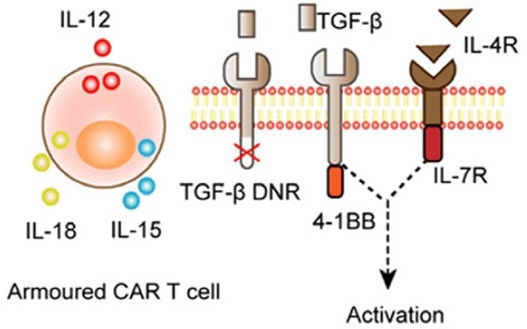
Figure 2. Armored CAR-T cell that utilizes self-secretion of cytokines to promote immune activation within the TME. 2 Image Credit: ACROBiosystems
The TME also manipulates immune checkpoints, which promotes the immunosuppressive environment. T cell efficacy is adversely affected by the expression of these immunosuppressive receptors on activated T cells, which have a high affinity to ligands expressed on target cells.
Immunosuppressive cellular signals are promoted as a result of these manipulated immune checkpoints. An approach that can overcome this issue involves gene editing technology that reprograms T cells, knocking out the immune checkpoint completely.
Studies have employed electrotransfection encoding using Cas9 and ShRNA knockout of PD-1 on modified T cells. This approach caused significantly enhanced cytotoxic effects. Similar results were achieved by knocking out CTLA-4 in cytotoxic T cells using CRISPR/Cas9, enhancing tumor growth inhibition and elimination.
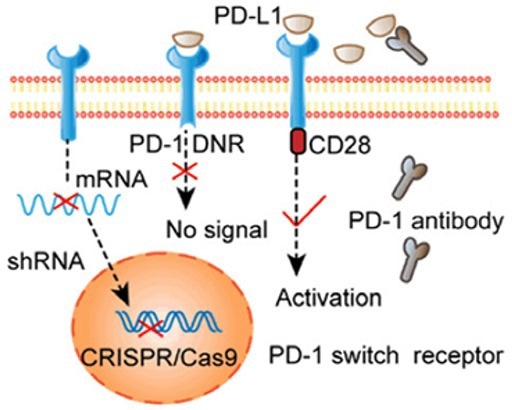
Figure 3. Selective activation of PD-1 signaling through electrotransfection encoded sgRNA and Cas9 knockout PD-1 T cells. 2 Image Credit: ACROBiosystems
Precision targeting of tumor cells by tumor-specific antigens
Specifically targeting antigens expressed on the surface of tumors is a common modality used in treating solid tumors. However, due to the heterogeneity of tumor cells in solid tumors, a diverse set of antigens is commonly present. This leads to ineffective immune surveillance, promoting recurrent tumors.
Targets, including GPC3, HERS, and MSLN, are common in tumor tissue, with favorable clinical results reported in current studies. CAFS also possess reported angiogenesis capabilities, and play a key role in solid tumor progression and metastasis.
The safety and precise activity of modified T cells is directly determined by the antigen specificity of the CAR structure. Current targets possess reasonably high tumor specificity in some solid tumors. For this reason, they have become a focus of CAR-T research, achieving favorable clinical outcomes.
One specific protein which is highly expressed in CAFs is FAP, which represents a promising target for reducing tumor growth and matrices, and has vast potential in the development of the next generation of CAR-T cells and cell therapy techniques.
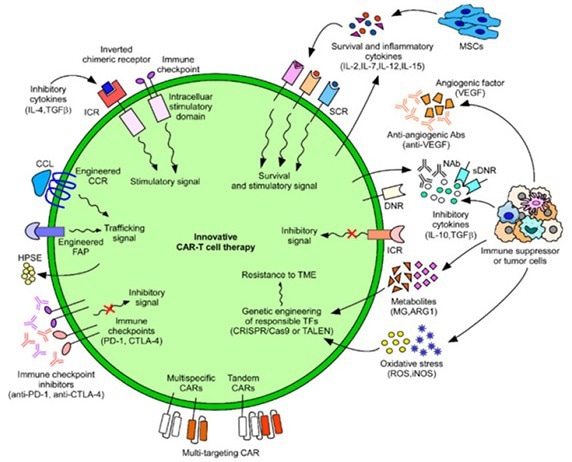
Figure 4. Innovative CAR-T cell therapies developed to combat cancers. 3 Image Credit: ACROBiosystems
Conclusion
There are numerous challenges with using CAR-T cell therapy for the effective treatment and eradication of solid tumors. However, the development of several innovative strategies in recent years has raised the hopes of vastly improving clinical outcomes for cancer patients.
Current research into the optimization and modification of CAR cell structure alongside various ancillary therapies provides a potentially revolutionary breakthrough in cell therapy and the treatment of solid tumors.
Contact the experts
ACROBiosystems has developed a range of target proteins for solid tumor cell therapy and research, including FAP, GPC3, MSLN, EGFRvIII, and HER2. Proteins provided by the company cover different fluorescent types and species. Products can be used for CAR expression, immune screening, clinical PK, and other studies.
Contact the experts at ACROBiosystems today to find out more about their range of solutions for cell therapy studies.
References and Further Reading
- Hou A., et al. (2021). Navigating CAR-T cells through solid-tumor microenvironment. Nature Rev. Drug Discov.. 20(7):531-550.
- Huang M., et al. (2020). Innovative strategies to advance CAR T cell therapies for solid tumors. Am J Cancer Res. 10(7):1979-1992
- Jo Y., et al. (2020) Innovative CAR-T Cell Therapy for Solid Tumor; Current Duel between Car-T Spear and Tumor Shield. Cancers (Basel). 12(8):2087.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.