
Image Credit: ACROBiosystems
What is Parkinson’s Disease?
Parkinson’s disease (PD) is the second most prevalent neurological disease that affects middle-aged and elderly people. Resting tremors, stiffness, bradykinesia, impaired gait, and posture are the most common clinical signs.
Parkinson’s disease is more common as people become older; the typical starting age is around 60 years old, and only around 4% of PD patients are diagnosed just before age of 50. Men are also 1.5 times more likely than women to develop Parkinson’s disease. According to published studies, Parkinson’s disease affects about 1.7% of China’s population over the age of 65. Furthermore, the bulk of Parkinson’s cases arises at random, with just around 10% having a genetic link.
According to Frost & Sullivan statistics, the number of individuals over 65 in China with Parkinson’s disease continues to rise, reaching 2,831,000 in 2018. In 2023, this number is expected to rise to 3,459,000. According to the most recent research, the number of PD cases in the United States is predicted to rise to over 1 million by 2030. As a result, there is a significant need for a unique and effective therapy for Parkinson’s disease sufferers all over the world.
Etiology and pathological hallmarks of PD
The etiology of Parkinson’s disease is unknown; the risk of getting the disease is dependent on the interaction of genetic and environmental risk factors. Deterioration of dopaminergic neurons in the substantia nigra pars compacta (SNc) and aberrant aggregation of alpha-synuclein (SNCA), the primary component of Lewy bodies, are two pathological hallmarks of Parkinson's disease.
SNCA
SNCA is a presynaptic protein of 140 amino acids that plays a role in neural plasticity, membrane vesicle preparation, and neurotransmitter release. An N-terminal lipid-binding domain, a core non-beta-amyloid component, and also an acidic C-terminal domain make up SNCA.
Multiple neurological dysfunctions and degeneration pathways have been linked to abnormal SNCA, including inflammation, decreased mitochondrial function, changed protein degradation systems, and oxidative stress.
Normal SNCA is found in the form of unfolded monomers. In Parkinson’s disease, SNCA experiences inappropriate post-translational changes, such as phosphorylation at the Ser129 site, causing it to fold into dimers, trimers, and oligomers, however, it is unclear which polymerization form causes neurotoxicity. These polymers then clump together to form protofibrils and amyloid fibrils, which build up inside neurons, limiting function and eventually leading to neuron death.
In both sporadic and familial PD, SNCA has emerged as a significant target for creating novel PD treatments.
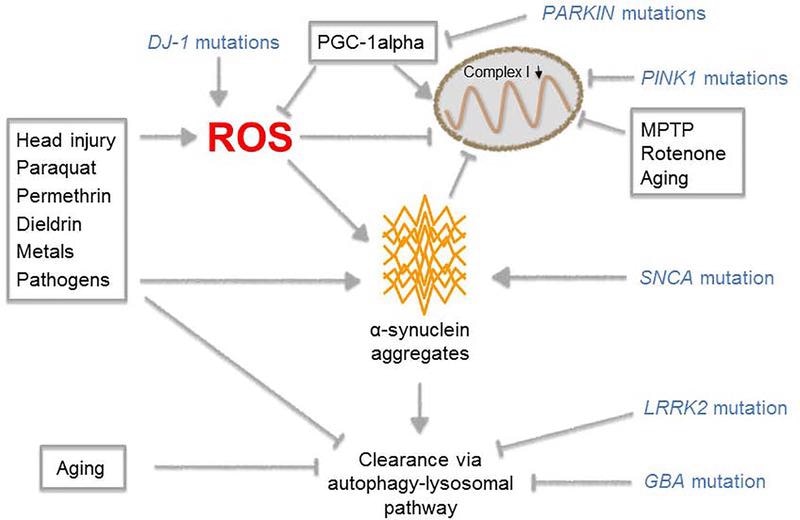
Multiple pathways that influence the onset of PD. Image from reference
Advances in the development of drugs targeting SNCA
Currently, therapies aimed at reducing SNCA levels directly or indirectly, as well as modulating the inflammatory process, are being developed. Immunotherapy for SNCA can take the form of passive or active vaccination.
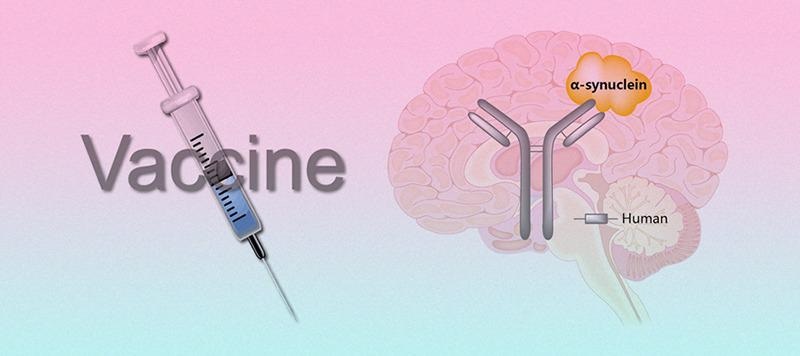
Immunotherapy targeting SNCA. Image from reference
Active immunization is a traditional vaccination approach in which SNCA antigens are used as immunological stimulants to promote a long-lasting humoral response and specific antibody formation.
Table 1. Source: ACROBiosystems
| Drug Name |
Status |
Indications |
Company |
| ACI-7104 |
Phase I |
Parkinson's Disease |
Ac Immune |
| UB-312 |
Phase I |
Parkinson's Disease;
Parkinsonism |
United Neuroscience |
| Affitope-PD01 |
Phase I |
Multiple Sclerosis;
Parkinson's Disease;
Neurodegenerative disease |
Affiris |
| PV-1950 |
Preclinical |
Parkinson's Disease |
Institute For Molecular Medicine;
Nuravax |
| Affitope-PD03 (AFFiRiS) |
No advance |
Multiple Sclerosis;
Parkinson's Disease;
Neurodegenerative disease |
Affiris |
Several anti-SNCA antibody drugs are currently undergoing clinical trials in Phase II, Phase I, and preclinical phases in passive immunotherapy. Cinpanemab/BIIB054 was shown to be safe and tolerated in the Phase I clinical study; however, the Phase II clinical trial was stopped owing to safety concerns that did not satisfy the primary and secondary objectives.
Due to the different binding sites of SNCA, and as Cinpanemab binds to the N terminus of SNCA and Prasinezumab attaches to the C terminus, Prasinezumab appears to provide beneficial effects.
Table 2. Source: ACROBiosystems
| Drug Name |
Target |
Drug/Therapy Type |
Status |
Indications |
Company |
| Prasinezumab |
SNCA |
Humanized monoclonal antibody |
Phase II |
Parkinson's Disease |
Prothena |
Lu AF-82422
(Lundbeck A/S) |
SNCA |
Monoclonal antibody |
Phase II |
Multiple Sclerosis;
Parkinson's Disease |
Lundbeck;
Genmab |
| UCB-7853 |
SNCA |
Monoclonal antibody |
Phase I |
Parkinson's Disease |
Ucb Biopharma Srl |
| MEDI-1341 |
SNCA |
Antibody |
Phase I |
Parkinson's Disease |
AstraZeneca plc;
Takeda Pharmaceutical Co Ltd |
| Anti-a-syn antibody |
SNCA |
Antibody |
Pre-clinical |
Parkinson's Disease |
Ac Immune |
| ATV:aSyn |
SNCA |
Antibody |
Pre-clinical |
Parkinson's Disease |
Denali Therapeutics Inc |
| ABL-301 |
IGF1R;
SNCA |
Bispecific antibody |
Pre-clinical |
Parkinson's Disease |
Abl Bio |
| PR-004 |
GlcCer;
SNCA |
Genetic therapy |
Pre-clinical |
Neurodegenerative disease |
Eli Lilly |
Existing research continues to fall short of meeting the clinical demands of Parkinson’s disease treatment. The underlying neuropharmacology of symptoms is minimally understood in comparison to motor symptoms. Furthermore, the predictive usefulness and effective application of preclinical models have not been adequately investigated, and clinical studies frequently lack novel evaluable drugs.
Scientific research and technology innovation is intended to improve symptomatic and disease-modifying treatment for Parkinson’s disease patients.
To aid with the study and development of Parkinson’s disease therapeutic medications, ACROBiosystems has SNCA/Alpha-Synuclein protein (Met 1 - Ala 140).
Table 3. Source: ACROBiosystems
| Cat. No. |
Species |
Product Description |
| ALN-H52H8 |
Human |
Human Alpha-Synuclein Protein, His Tag |
| ALN-H82H8 |
Human |
Biotinylated Human Alpha-Synuclein Protein, His, Avitag™ |
High-purity SNCA/Alpha-Synuclein proteins are verified using SDS-PAGE.
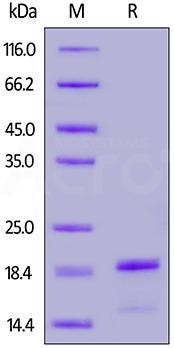
Purity>90%(Cat. No. ALN-H52H8). Image Credit: ACROBiosystems

Purity>95%(Cat. No. ALN-H82H8). Image Credit: ACROBiosystems
Other strategies for PD treatment
LRRK2
The most important genetic risk factor for PD is the leucine-rich repeat kinase 2 (LRRK2) gene, which generates the most prevalent monogenic variants of the disease. LRRK2 is a kinase that controls the activity of other proteins by phosphorylating them. LRRK2 kinase enzymatic activity is increased by pathogenic mutations.
The RAB GTPase subgroup, which regulates cellular processes, such as vesicular transport, cellular breakdown pathways, and immunological and microglial cell responses, is one of LRRK2’s regulatory targets. Small molecule LRRK2 kinase inhibitors are neuroprotective in preclinical PD models, making LRRK2 one of the most relevant targets for PD therapy development.
Targeting dopamine-related pathways: DRD1, DRD2, DRD3, MAO-B, DDC
Motor symptoms in Parkinson’s disease are caused by the death of dopaminergic neurons in the substantia nigra. When these neurons die, the basal neuromotor circuit becomes dysregulated, affecting motor integration, execution and process control.
When the function of the substantia nigra is damaged, it is unable to provide proper reciprocal inhibition of the active and antagonistic muscles, resulting in uncoordinated irregularities in the active and antagonistic muscles and the onset of Parkinson’s symptoms.
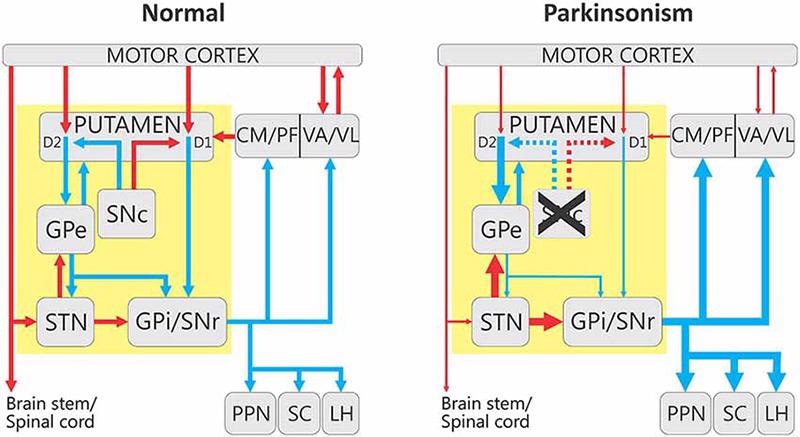
Changes in the basal ganglia-thalamocortical motor circuit in parkinsonism. Image from reference. Image Credit: Frontiers in Neuroanatomy
A key technique for treating Parkinson’s disease is to target a dopamine-related pathway. Overall, enhanced dopamine binding to receptors and dopamine pathway activity may be useful in reducing Parkinson’s symptoms.
- In clinical applications, levodopa drugs are the most often used drugs for PD therapy. These drugs can pass across the blood-brain barrier and convert to dopamine in the brain. Dopamine receptor agonists, which imitate dopamine’s activity and bind to the DRD1, DRD2 and DRD3 dopamine receptors, can also be used for therapy.
- DOPA decarboxylase (DDC) is a catalytic enzyme that catalyzes the decarboxylation of dopa to generate dopamine (i.e., hydroxytyramine). Although levodopa may penetrate the blood-brain barrier, only 1-5% of dopaminergic neurons enter the brain, and most of the levodopa is digested by peripheral DDC before reaching the brain; hence, DDC inhibitors are frequently given together with it. DDC inhibitors that do not penetrate the blood-brain barrier are frequently used with levodopa to raise levodopa levels in the central nervous system.
- Monoamine oxidase B (MAO-B) is a key enzyme in the breakdown of neurotransmitters including dopamine. The MAO inhibitor (MAOI) family of drugs works by blocking the action of MAO-B.
Table 4. Source: ACROBiosystems
| Molecule |
Cat. No. |
Species |
Product Description |
| DDC |
DDC-H55H6 |
Human |
Human DDC/Dopa Decarboxylase Protein, His Tag |
| MAOA |
MAA-M5547
|
Human |
Human MAOA Protein, His Tag (active enzyme) |
| MAOA |
MAA-M5548 |
Mouse |
Mouse MAOA Protein, His Tag |
| MAOB |
MAB-H5547 |
Human |
Human MAOB / Monoamine Oxidase B Protein, His Tag (active enzyme) |
References
- S.H. Fox, J.M. Brotchie. Special Issue on new therapeutic approaches to Parkinson disease. Neuropharmacology(2022). https://doi.org/10.1016/j.neuropharm.2022.108998
- Fleming, S.M., Davis, A., Simons, E. Targeting alpha-synuclein via the immune system in Parkinson’s disease; current Vaccine therapies Neuropharmacology(2022). https://doi.org/10.1016/j.neuropharm.2021.108870
- Simon, D. K., Tanner, C. M., & Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clinics in geriatric medicine (2020). https://doi.org/10.1016/j.cger.2019.08.002
- Galvan, A., Devergnas, A., & Wichmann, T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state (2015). https://doi.org/10.3389/fnana.2015.00005
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.