Pharmacokinetics (PK) is a branch of science that deals with the quantitative analysis of distribution, absorption, metabolism, and egesting of drug molecules inside the body of a living organism.
All pre-clinical and clinical studies involve the measurement of serum drug concentration, in animals as well as in patients, at various stages after drug administration. The outcome is a key indicator of the pharmacokinetic properties of the drug and is pertinently relevant to dosing recommendations.
The flourishing biologic drugs market, steered by a spurt of success with monoclonal antibodies, has led to the need for standard high-throughput assays to assess the content of mAbs in serum samples. Conventionally, the analyses are carried out through indirect ELISA or by using anti-drug antibodies (ADA), each with its own merits and demerits.
ACROBiosystems has developed a range of assays based on the competitive ELISA method. A biotinylated mAb against the same target is used as a tracer by the assay to compete with the unlabeled analyte, and the interaction between streptavidin and biotin is used for the final readout. This technique is not dependent on ADAs, and it also reduces the background problem that occurs frequently with the conventional assay format.
ACROBiosystems has introduced kits for the analyses of CTLA-4, PD-1, and HER-2, in humans as well as common experimental animals.
Product List
| Cat. No. |
Product Description |
Size |
| EPH-V1 |
ELISA Assay Kit for Anti-PD-1 h-mAb in Human Serum |
96/480 tests |
| EPM-V1 |
ELISA Assay Kit for Anti-PD-1 h-mAb in Mouse Serum |
96/480 tests |
| EPC-V1 |
ELISA Assay Kit for Anti-PD-1 h-mAb in Monkey Serum |
96/480 tests |
| EHH-V1 |
ELISA Assay Kit for Anti-HER-2 h-mAb in Human Serum |
480 tests |
| EHM-V1 |
ELISA Assay Kit for Anti-HER-2 h-mAb in Mouse Serum |
480 tests |
| EHC-V1 |
ELISA Assay Kit for Anti-HER-2 h-mAb in Monkey Serum |
480 tests |
| ECH-V1 |
ELISA Assay Kit for Anti-CTLA-4 h-mAb in Human Serum |
96/480 tests |
| ECM-V1 |
ELISA Assay Kit for Anti-CTLA-4 h-mAb in Mouse Serum |
96/480 tests |
| ECC-V1 |
ELISA Assay Kit for Anti-CTLA-4 h-mAb in Monkey Serum |
96/480 tests |
Product Features
Low Background
The serum samples include several factors that may possibly interfere with the indirect ELISA result. This is the main reason for the background problem (Figure 1A). Thus, a series of testing should be carried out to determine the minimum required dilution (MRD) prior to performing an experiment. This can take a long time and the results can fluctuate. Conversely, the competitive ELISA method used for the kit does not have a background problem. As demonstrated in Figure 1B, dilutions up to 1:5 do not generate any background at all.
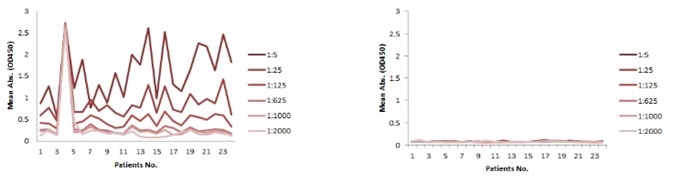
Figure 1. Background comparison between indirect and competitive ELISA with samples of different dilutions. A: Indirect ELISA; B: Competitive ELISA. (Serum sample from Cancer Institute & Hospital. Chinese Academy of Medical Sciences GCP Centre).
Application
It is possible to use the assay kits for analyses of any mAbs that share comparable binding domain just like the tracer biotinylated antibody.
For instance, with their Anti-PD-1 mAb kit for human serum samples (Cat. No. EPH-V1), the company has successfully quantified five different anti-PD1 mAbs that are either being tested in clinical trials or already available on the market.
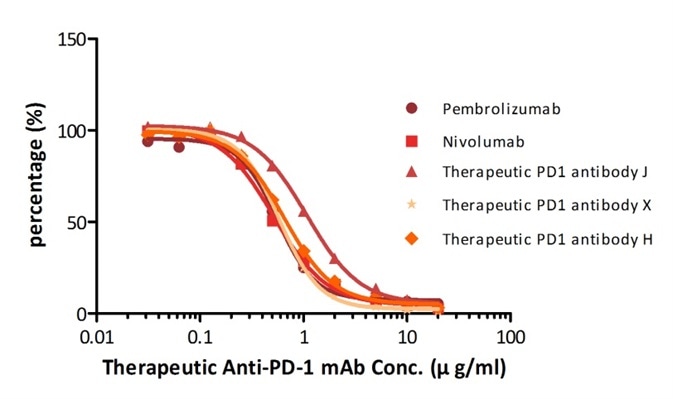
| Name |
Pembrolizumab |
Nivolumab |
Therapeutic PD-1 Antibody J, Human IgG4 |
Therapeutic PD-1 Antibody X, Human IgG4 |
Therapeutic PD-1 Antibody H, Human IgG4 |
| Detection Range (μg/ml) |
0.03125-20 |
0.03125-20 |
0.03125-20 |
0.03125-20 |
0.03125-20 |
| Sensitivity (μg/ml) |
0.15625 |
0.15625 |
0.15625 |
0.15625 |
0.15625 |
| %Recovery |
88-113 |
92-114 |
86-114 |
96-112 |
87-106 |
Figure 2. Determination of serum drug concentration for five PD-1 therapeutic antibodies using PD-1 ELISA Kit.
High Batch-to-Batch Consistency
ACROBiosystems establishes stringent quality control program to guarantee the lot-to-lot consistency of their products. Each set of products is examined against the internal standards using different analytical approaches. The product is released only if it conforms to all standards.
High Stability
All kits components are tested for their stability using the accelerated testing method. On the basis of the results represented in Figure 3, the products can be stored at −80 °C for 4–6 months. In addition, the assay components are analyzed after one or two freeze-thaw cycles. Under both conditions, there was no considerable activity loss.
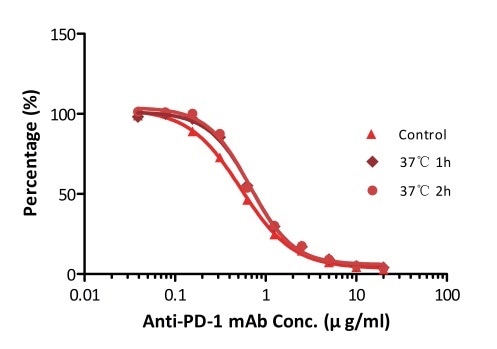
Figure 3. ELISA using Anti-PD-1 h-mAb kit Human Serum (Cat. No. EPH-V1). The samples were incubated at 37 °C for 2 hours after reconstitution. No significant loss of activity was observed.
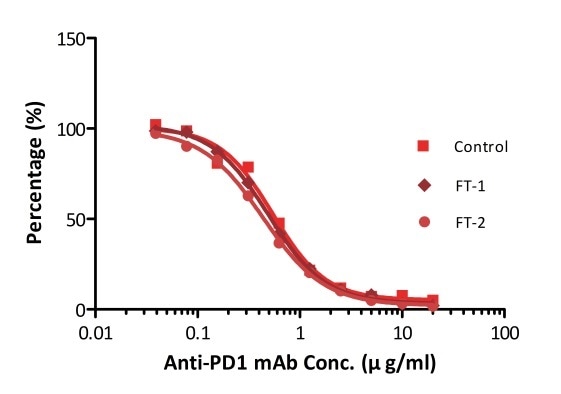
Figure 4. ELISA using Anti-PD-1 h-mAb kit Human Serum (Cat. No. EPH-V1). The samples were subjected to zero, one, and two rounds of freeze-thawing cycles, respectively. No significant loss of activity was observed.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.