At the American Association for Cancer Research (AACR) 2022 Annual Meeting, Immune-Onc Therapeutics released their research of io-108, a novel clinical-stage myeloid checkpoint inhibitor targeting LILRB2 (ILT4). Well-tolerated in the ongoing Phase 1 study for the treatment of solid tumors, IO-108 promotes innate and adaptive anti-cancer immunity in preclinical models.
A new target for drug development: Leukocyte immunoglobulin-like receptor Bs (LILRBs)
The Leukocyte Immunoglobulin-like Receptor family (LILRs) contains members that are critical to the regulation of immune response, such as cell proliferation, cell migration, cytokine production, phagocytosis, and secretion, chemical mediator production and secretion, and disease progression.
LILRBs 1-5 are inhibitory LILRs, while the most extensively investigated are LILRB1, also known as LIR1, ILT2, and CD85J) and LILRB2, which is also known as LIR2, ILT4, and CD85D).
Different types of leukemia and solid tumors express LILRB1, which prevents primary cutaneous T cell lymphoma cell death and augments gastric tumor growth, while neoplastic B cell proliferation is inhibited by HLA-G/LILRB1 interaction.
ITL4, otherwise known as LILRB2, is predominantly expressed on myeloid cells, which include dendritic cells, macrophages, monocytes, and neutrophils. LILRB2 can interact with various ligands such as HLA-G, Angiopoietin-like proteins (ANGPTLs), SEMA4A, and CD1d in a tumor microenvironment (TME) in solid tumors to promote tumor immune escape and myeloid tumor proliferation.
HLA-G: A new target for cancer immunotherapy
HLA-G, otherwise known as Human Leukocyte Antigen G, plays an essential role in fetal-maternal tolerance and is a non-classical MHC class I molecule that contributes to immune escape or anergy. Recently, it has been considered an immune checkpoint molecule.
To date, there are seven subtypes of the HLA-G protein, including HLA-G1, G2, G3, and G4, which are four membrane-bound isoforms, and HLA-G5, G6 and G7, which are three secreted soluble isoforms.
The full-length molecule HLA-G1 and its soluble isoform HLA-G4 have occupied the most research. Cytotoxic T cells are obstructed by HLA-G, along with natural killer (NK) cells and B cells. HLA-G also induces T cell anergy, regulates myeloid cells, and promotes T regulatory cells (Tregs), in addition, to being expressed on antigen-presenting cells (APCs).
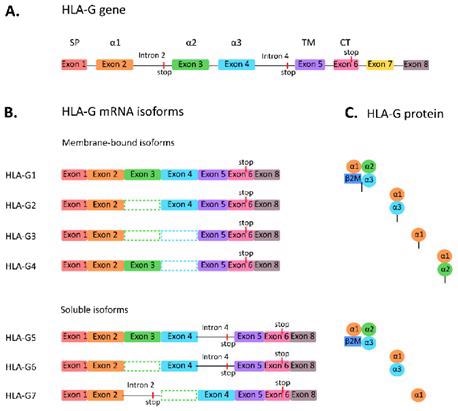
Figure 1. Subtypes of HLA-G. Image Credit: ACROBiosystems
LILRBs interact with HLA-G to promote immune escape
Studies have recently shown that the HLA-G/LILRBs pathway has a wider impact than CTLA-4 and PD-1/PD-L1 regulated immune cells. It has been proven that the interaction of HLA-G with LILRB1 inhibits the function of NK cells, dendritic cells, monocytes/macrophages, T cells, and B cells and therefore aids tumor escape.
It has also been discovered that the interaction between LILRB2 expressed on solid tumor cells and HLA-G on adjacent tumor cells upregulates the expression of vascular endothelial growth factor C (VEGF-C), causing metastasis, proliferation, and lymphangiogenesis through the extracellular signal-regulated kinase (ERK) signaling pathway.
Tumor escape is also encouraged by the B7-H3 expression via PI3K/AKT/mTOR signaling, which is itself in turn upregulated by this interaction.
Cytotoxicity is inhibited by the interaction between LILRB1-expressing natural killer (NK) cells and HLA-G expressed on tumor cells and CD8+ T cells, which promotes tumor escape. Differentiation into Th2 cells and regulatory T cells (Tregs) is also induced by the expression of LILRB1 on naïve T cells.
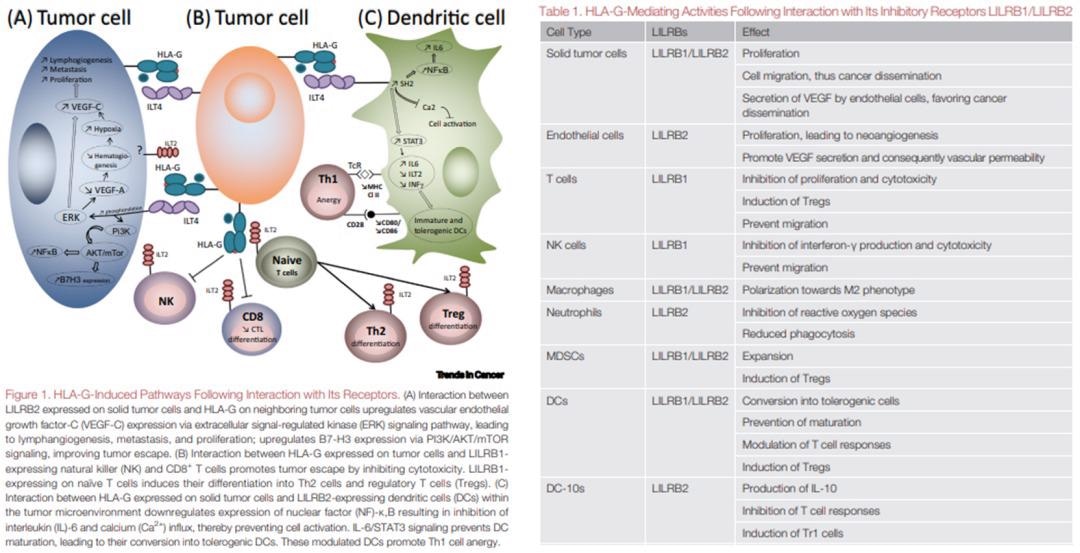
Figure 2. LILRBs interact with HLA-G to induce immunosuppression and promote tumor cell proliferation. Image Credit: ACROBiosystems
Prolonged antigen stimulation causes antigen-specific T-cells to become dysfunctional, leading to the induction of depleted T cells during tumor development. Poor effector function and expression of PD-1, TIM-3, LAG-3, CTLA-4, and TIGIT characterizes T cell exhaustion.
Targeting CTLA-4 or PD-1 on T cells in certain tumor micro-environments may be an effective option in specific cancer treatment. There is, however, undeniably a risk of tumor-infiltrating T cells becoming refractory to checkpoint-blockade-mediated reactivation.
A population of tumor-infiltrating CD8+ T cells expressing LILRB1, separate and distinct from CD8+PD1+ T cells was recently identified in ccRCC patients. HLA-G expression prevents the CD8+LILRB1+-mediated cytotoxicity, which is subsequently neutralized by anti-HLA-G antibodies, which validates the notion that targeting HLA-G can rejuvenate exhausted cancer-infiltrating T cells.
It has been demonstrated that LILRB1/2 has more affinity to HLA-G1 dimer, which results in increased inhibitory signals. A single monomer is bound to a single LILRB1/2 molecule by the HLA-G1 monomer, whereas the HLA-G1 dimer is able to simultaneously interact with two LILRB1/2 molecules, which then amplify the LILRB-associated inhibition signals.
Selecting potential novel targets and forging a unique, visionary path can help innovators stand out as the competition and development in cutting-edge drug research intensifies. The next hot immunotherapy targets may be LILRB and HLA-G.
ACROBiosystems has released a high-quality HLA-G tetramer protein (Cat. No. HLG-H52E9), which can be used for immunosuppression research of LILRBs and HLA-G pathways to facilitate related drug development.
Product features
- Tetramer proteins have been developed based on ACROBiosystems’ unique biotin labeling technology. It has been shown that these tetramer proteins amplify cell signals, stronger activities, and improved inhibitory effects on LILRB and HLA-G pathways.
- Purity is verified by SDS-PAGE (> 95%) and SEC-MALS (> 85%), and the tetramer structure is verified by reducing and non-reducing electrophoresis and SEC-MALS.
- ELISA assay neutralizes bioactivity. ACROBiosystems has also released a series of high-quality LILRBs proteins for antibody screening, immunization, and species cross-verification, along with other applications.
A series of high-quality LILRBs proteins for immunization, antibody screening, species cross-verification, and other applications have also been released.
Product features
- There are multiple species available, including Human, Mouse, and Cynomolgus species, to be used in species cross-verification.
- High-purity: The purity is verified by SDS-PAGE (>95%) and MALS (>90%).
- Bioactivity is verified by ELISA and SPR assays, whilst affinity is verified by antibody and ligand binding assays.
Product list
Assay data
Homogenous aggregate structure and purity by MALS verification
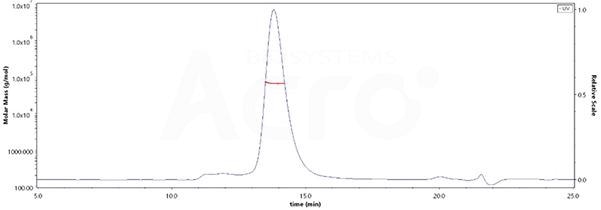
Image Credit: ACROBiosystems
When it comes to the purity of Human LILRB2, His Tag (Cat. No. LI2-H5220) is more than 90%, whilst its molecular weight is around 58-78 kDa verified by SEC-MALS.
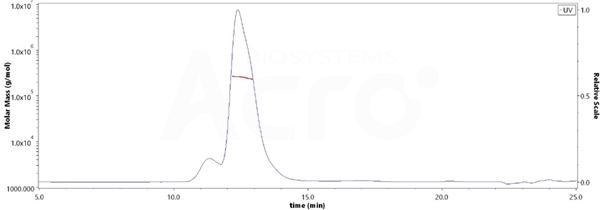
Image Credit: ACROBiosystems
Regarding the purity of Human HLA-G RIIPRHLQL Tetramer Protein (Cat. No. HLG-H52E9), this is over 85% and the molecular weight of this protein is around 228-278 kDa verified by SEC-MALS.
High bioactivity by ELISA verification
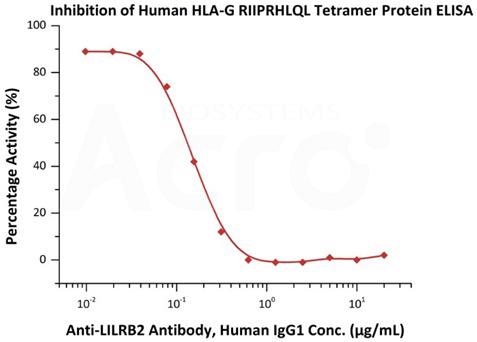
Image Credit: ACROBiosystems
Serial dilutions of Anti-LILRB2 Antibody, Human IgG1 were added into Human LILRB2, Fc Tag (Cat. No. CDD-H5259) : Human HLA-G RIIPRHLQL Tetramer Protein (Cat. No. HLG-H52E9) binding reactions. The half maximal inhibitory concentration (IC50) was recorded at 0.1486 μg/mL (QC tested).
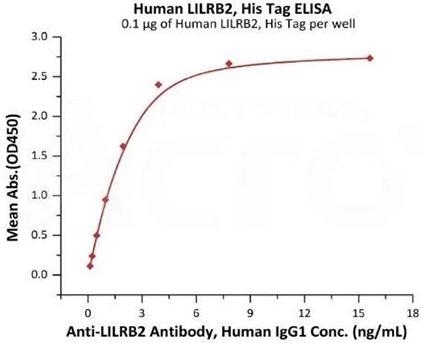
Image Credit: ACROBiosystems
Immobilized Human LILRB2, His Tag (Cat. No. LI2-H5220) at 1 μg/mL (100 μL/well) can bind Anti-LILRB2 Antibody, Human IgG1 with a range of 0.5-4 ng/mL (QC tested).
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.