There is a growing use of biomolecular interaction analysis in drug development to kinetically define the binding activities of molecules. ACROBiosystems provides SPR /BLI analytical services to its valued customers, which is based on the Biacore T200/8K platform and ForteBio Octet platform. The SPR/BLI analytical services incorporate kinetic analysis, interaction measurement, antibody pairing/group, and antibody screening.
Maintaining a dependable, strong, and professional technical team, ACROBiosystems offers first-rate integrated SPR&BLI analytical services. ACROBiosystems has collaborated with and served over 100 customers in both industry and academia. ACROBiosystems’ work has assisted a multitude of antibody drugs IND applications.
This article details how SPR&BLI technology provided by ACROBiosystems Detection and Analysis Service Center can support you throughout the entire process for antibody drug development.
Case study
1. High-throughput antibody screening
Performing an initial affinity screening of antibodies is useful for ranking the candidates based on their specificities as well as kinetics. This can speed up the entire therapeutic screening process.
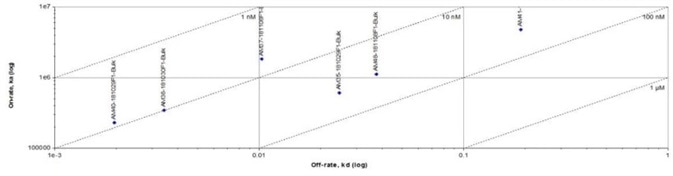
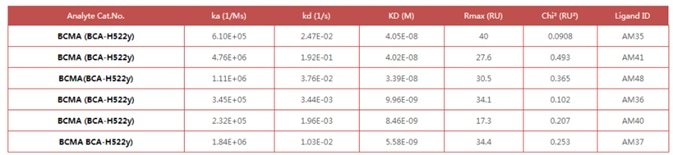
Figure 1. Anti- BCMA antibodies (Mouse IgG1) captured on CM5 chip via anti-Mouse IgG antibody, can bind Human BCMA Protein, His Tag (Cat. No. BCA-H522y) as screened assay in an SPR (Biacore T200). Image Credit: ACROBiosystems
2. Antibody characterization
Based on the multiple concentration affinity testing result, the potential to understand the affinity and kinetic activities of the antibody and accurately forecast the metabolism of the drug in vivo becomes clear.
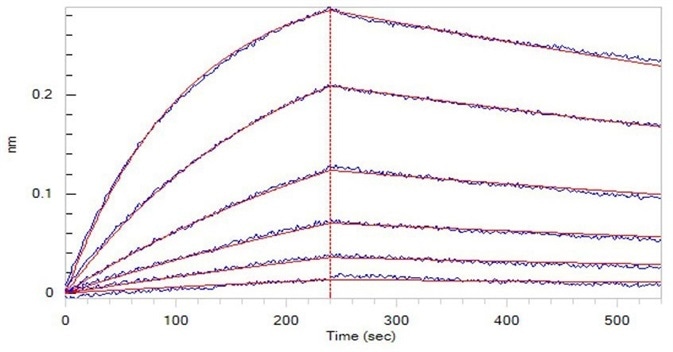
Figure 2. Opdivo (Nivolumab), loaded on Protein A biosensor, can bind to Human PD-1, His Tag (HPLC-verified) (Cat. No. PD1-H5221) with an affinity constant of 5.09 nM as determined in BLI assay (ForteBio Octet Red96e). Image Credit: ACROBiosystems
3. Batch-to-batch consistency evaluation
Utilization of SPR technology is possible for quality control and batch-to-batch consistency throughout the whole therapeutic production process.
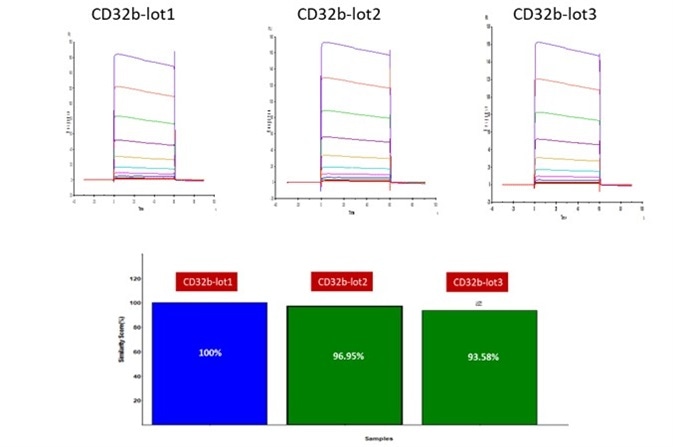
Figure 3. Batch-to-batch consistency result of Human Fc gamma RIIB / CD32b (Cat. No. CDB-H5228). CV is within 10% (Biacore T200). Image Credit: ACROBiosystems
4. Detection of affinity between the antibody and Fc receptor protein
The efficacy of therapeutic antibodies relies on the binding between the Fab fragments and target antigen, as well as the binding between their Fc fragments and Fc receptors. Thus, it is critical to screen and optimize antibodies with the best affinity to Fc receptors in order to develop therapeutic monoclonal antibodies.
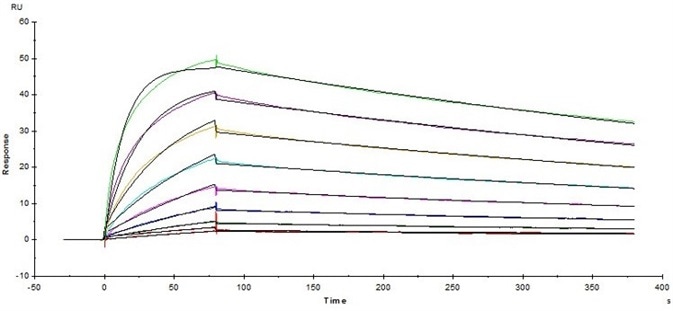
Figure 4. Immobilized Human Fc gamma RI / CD64 Protein (Cat. No. FCA-H52H2) on CM5 Chip via anti-Histidine antibody, can bind Herceptin® with an affinity constant of 5.45 nM as determined in SPR assay ( Biacore T200 ). Image Credit: ACROBiosystems
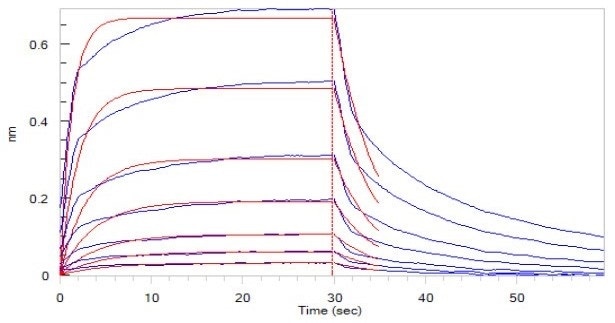
Figure 5. Human CD16a (F176), His Tag (SPR & BLI verified) (Cat. No. CDA-H5220) loaded on HIS1K Biosensor, can bind to Rituximab with an affinity constant of 1.72 μM as determined in BLI assay (ForteBio Octet Red96e). Image Credit: ACROBiosystems
5. Cross-species reactivity screening
Antibodies may have varying reactivities against antigens from different species. Therefore, cross-species reactivity screening assists in the determination of the most suitable animal model for any animal-based studies.
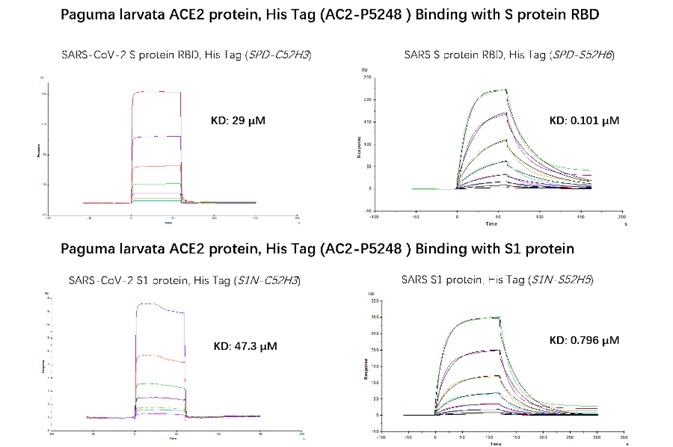
Figure 6. SPR verified that Paguma larvata ACE2 protein has a higher binding affinity to SARS than SARS-COV-2. The affinity constant of SARS S RBD and civet ACE2 can reach 0.101 µM, while the affinity constant of SARS-CoV-2 S RBD protein and civet ACE2 is only 29 µM. The affinity constant of SARS S1 protein and civet ACE2 can reach 0.796 µM, while the affinity constant of SARS-CoV-2 S1 protein and civet ACE2 is only 47.3 µM. Image Credit: ACROBiosystems
6. Bispecific antibodies
Bispecific antibody (BsAb) is a kind of antibody molecule that can synchronously bind to two contrasting antigens or two varying epitopes on the same antigen. Bispecific antibodies can apply the synergistic effect of two monoclonal antibodies and may be more beneficial than the combined use of two monoclonal antibodies. Additionally, for molecule optimization, it is necessary to conduct the affinity measurement of the two Fab ends to their respective targets.
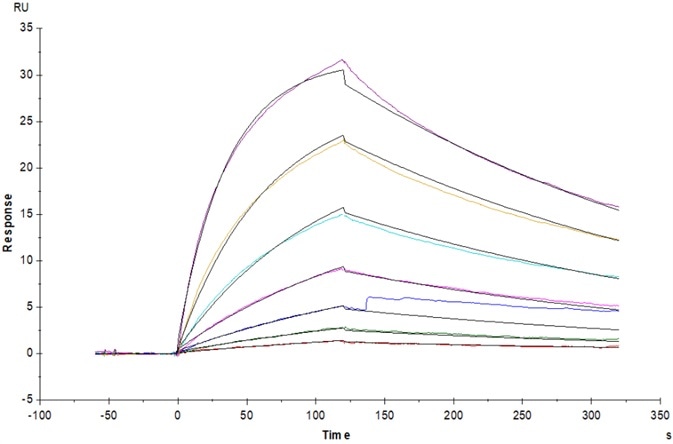
Figure 7. Bispecific T-cell Engager (CD3×BCMA) immobilized on CM5 Chip can bind to Human CD3E&CD3D Heterodimer Protein, His Tag&Tag Free (Cat. No. CDD-H52W1) with an affinity constant of 31.8 nM as determined in an SPR assay (Biacore T200). Image Credit: ACROBiosystems
7. Affinity measurement to complement systems
The majority of therapeutic antibodies kill or eradicate tumor cells through the CDC effect. The affinity between C1q protein complex and the antibody determines precisely whether it is possible to induce a strong CDC effect. Consequently, affinity testing to C1q protein is also vital in the continued development of antibody drugs.
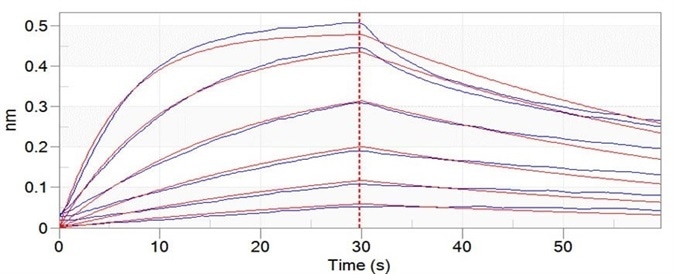
Figure 8. Trastuzumab can bind to C1q with an affinity constant of 3.24 nM as determined in BLI assay (ForteBio Octet Red96e). Image Credit: ACROBiosystems
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.