In 1973, interleukin 6 (IL-6) was detected as a soluble factor that is secreted by T cells. It is crucial for the generation of antibodies through B cells. The IL-6 signaling pathway has become a vital pathway that is involved in immune balance in health and immune dysregulation in several diseases.
IL-6 binds to its receptor IL-6 R, in both transmembrane and soluble forms, known as mIL-6R and sIL-6R, respectively. Later, the complex binds to the Glycoprotein 130 or gp130, and consequently, downstream signaling and gene expression are activated.
In the trans-signaling pathway, the sIL-6 complex with the receptor binds to gp130, and transduction of intracellular signal is started. In the subsequent step, the RAS-RAF, JAK-STAT and other pathways are stimulated, which promotes differentiation, cellular proliferation, immune regulation and oxidative stress.
The typical IL-6 signal is restricted to the cells (macrophages, neutrophils, T cells, etc.) that express IL-6R. However, when IL-6 levels are raised, the signal is commonly expressed due to the omnipresent nature of gp130.
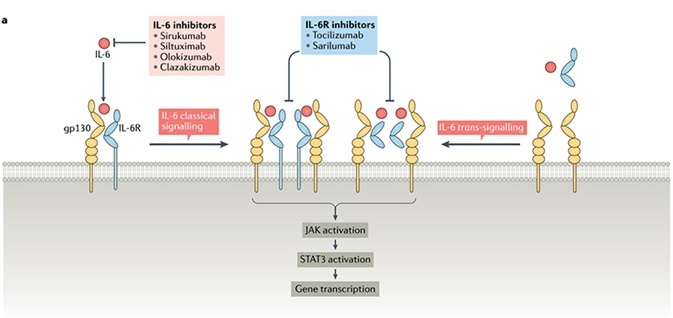
IL-6 signaling pathway. Image Credit: AcroBiosystems
With respect to diseases, IL-6 could have local inflammatory and systemic inflammatory effects. It is associated with the pathogenesis of different ailments, which include juvenile idiopathic arthritis (JIA), rheumatoid arthritis (RA), giant cell arteritis (GCA), adult-onset Still’s disease (AOSD), Castleman disease, cytokine release syndrome (CRS) and other such conditions.
In the dysfunction of the immune and inflammatory systems, IL-6 has several roles to play. Anti-IL-6 therapy could alleviate multiple symptoms — like fatigue, fever, pain, anemia and joint destruction.
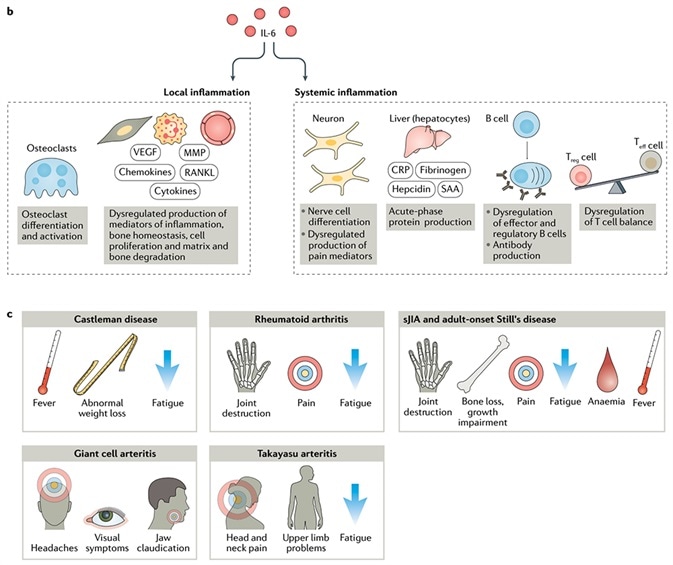
IL-6 has multiple roles in dysfunction of the immune and inflammatory systems. Image Credit: AcroBiosystems
When the team discovered that IL-6 signaling is facilitated via a high-affinity complex of IL-6R, IL-6 and gp130 hexamer, it proved challenging to use the traditional method to find small-molecule inhibitors.
Therefore, targeting the IL-6 or IL-6R, hindering the binding of IL-6 and IL-6R, eventually preventing IL-6 pathway signal transduction, the IL-6 pathway inhibitor takes on a new role as a potential treatment for several diseases.
At first, considering that receptor concentrations have low interpatient variability than IL-6 concentrations, likely simplifying the selection of dose and regimen, the team agreed to target IL-6R instead of IL-6.
Tocilizumab — the first humanized monoclonal antibody that targets IL-6R — hinders IL-6 signaling by inhibiting the binding of IL-6 with IL-6R. It has been accepted for several indications, such as pJIA, sJIA, RA, CRS, GCA and Takayasu arteritis (TA). Intravenous (IV) and subcutaneous (SC) preparations are included in Tocilizumab, and indications differ for different countries and regions.
Its IV formulation has been accepted for the treatment of sJIA, RA and CRS in China. In March 2021, the FDA permitted a new indication for Tocilizumab to delay the speed of lung function decline in adult patients who are suffering from systemic sclerosis-associated interstitial lung disease (SSc-ILD).
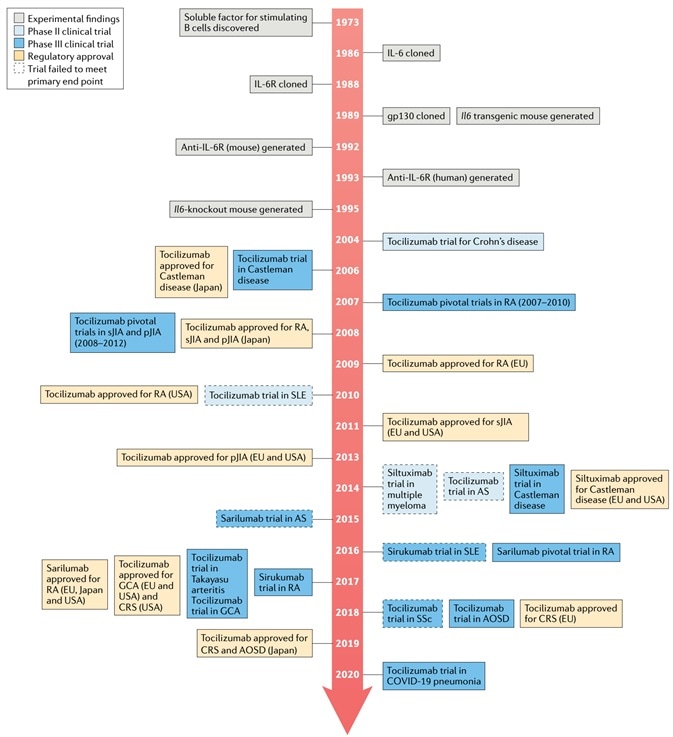
Advances in IL-6 and IL-6R targeted drugs. Image Credit: AcroBiosystems
IL-6 and CAR-T cell therapy
Tocilizumab was accepted by the FDA and EMA in 2017 and 2018, respectively, for treating serious or life-threatening CAR-T cell therapy-induced CRS in both children and adults (around 70% of patients cured with CD19 CAR-T cells will tend to develop CRS. CRS can bring about a fever, headache, chills, vomiting, severe nausea, diarrhea, musculoskeletal pain, hypotension, difficulty breathing, and tachycardia, which could be life-threatening in serious cases).
Authorization for this indication depends on information achieved from a retrospective analysis that shows the efficiency of Tocilizumab therapy in patients with CRS following CAR-T cell therapy in a potential clinical trial.
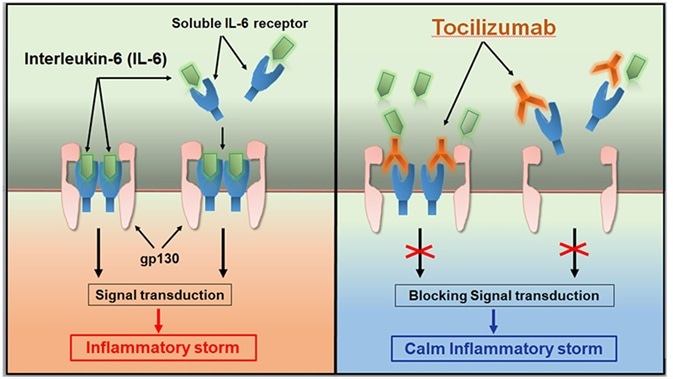
Tocilizumab calms the inflammatory storm. Image Credit: AcroBiosystems
The Tocilizumab’s therapeutic benefit as an IL-6R antibody has resulted in the development of multiple IL-6-targeting antibodies, like Olokizumab, Sirukumab and Clazakizumab; but, indications differ among them.
IL-6 and COVID-19
Earlier retrospective studies proposed that the COVID-19 epidemic progression might be due to CRS and revealed the IL-6’s possible role in critical COVID-19 patients.
The considerable increase in the IL-6 levels and other pro-inflammatory cytokines (such as IL-1, IL-8, IL-12) serves the main role in the COVID-19 patients’ deteriorating health condition.
Infectious T cells and inflammatory monocytes with the high secretion of IL-6 might get an entry in large numbers into the pulmonary circulation, provoke the inflammatory storm, and result in an immune disorder in critical COVID-19 patients.
It might encourage severe pneumonia to progress into acute respiratory distress syndrome, lead to immune dysfunction and consequently result in multisystem organ failure, and even high mortality.
Simply put, high concentrations of IL-6 create a larger cytokine storm that is unfavorable for the outcome of the diseases. Hence, for severe COVID-19 cases, IL-6 is a prospective therapeutic target.
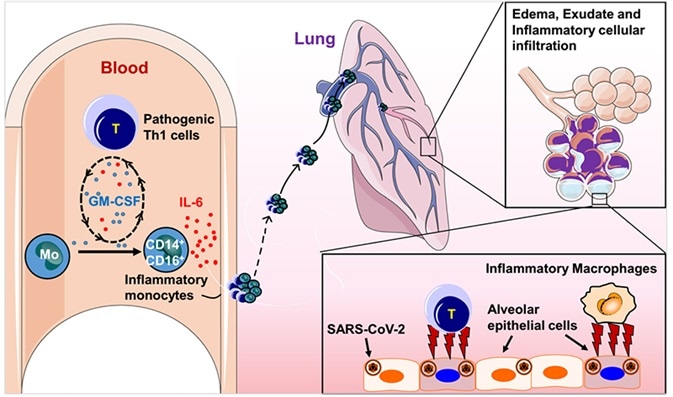
The pivotal role of IL-6 in the progression of COVID-19 patients. Image Credit: AcroBiosystems
To perform a potential meta-analysis of IL-6 antagonists in patients who are hospitalized for COVID-19, a prospective meta-analysis protocol was developed by the World Health Organization (WHO) Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group.
A total of 72 potentially suitable trials were found using systematic searches of ClinicalTrials.gov, the EU Clinical Trials Register, and the WHO International Clinical Trials Registry Platform from October 7th, 2020, to January 11th, 2021, out of which 27 (37.5 %) fulfill the selection criteria for the study.
The suitable trials randomly allocated COVID-19 hospitalized patients to a group in which IL-6 antagonists were administered and to a group in which no IL-6 antagonists or any other immunomodulators apart from corticosteroids were administered.
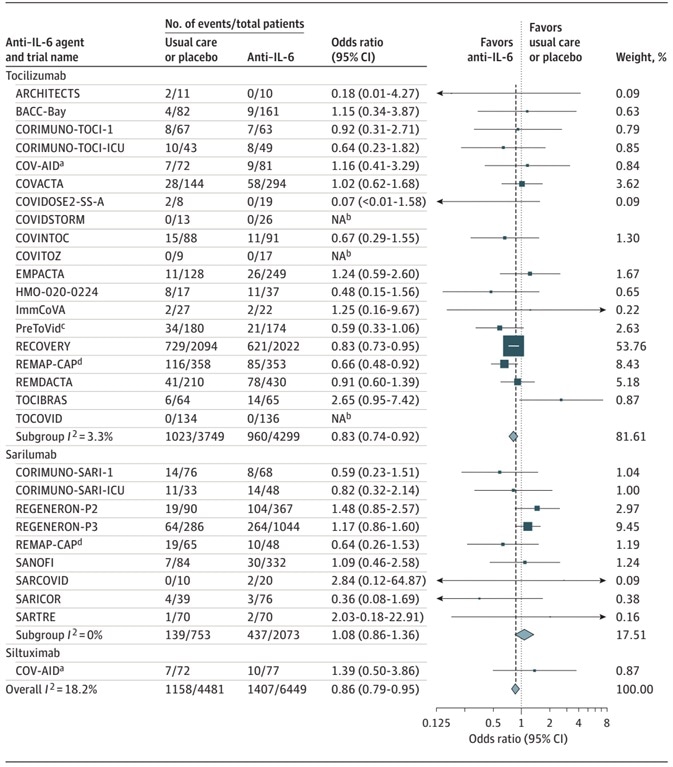
In total, 10,930 patients (with a median age of 61 years) participating in 27 trials were included. The main resulting measure was all-cause mortality at 28 days after randomization.
Among 6449 patients randomized to IL-6 antagonists, there were 1407 deaths by day 28. On the other hand, there were 1158 deaths out of 4481 patients who were randomized to usual care or placebo (summary OR, 0.86 [95% CI, 0.79-0.95]; P = 0.003 based on a fixed-effects meta-analysis).
The above analysis corresponds to 22% absolute mortality risk for IL-6 antagonists when compared with 25% assumed mortality risk for routine care or placebo. The summary ORs corresponds to 0.83 (95% CI, 0.74-0.92; P < .001) for Tocilizumab and 1.08 (95% CI, 0.86-1.36; P = .52) for Sarilumab.
The summary ORs corresponding to mortality compared with routine care or placebo in such receiving corticosteroids were 0.92 (95% CI, 0.61-1.38) for Sarilumab and 0.77 (95% CI, 0.68-0.87) for Tocilizumab.
When compared to usual care or placebo, the IL-6 antagonist was associated with a reduction in 28-day all-cause mortality.
Source: AcroBiosystems
Summary and prospect
Targeting the pathway of IL-6 paves way for groundbreaking treatments for different kinds of rheumatic diseases. Emerging indication proposes that IL-6 dysregulation contributes to different disease conditions, which include several types of cancer development, progression and metastasis.
On the other hand, high levels of IL-6 are linked with higher cancer risk and other conditions, like insulin resistance, coronary heart disease, asthma, advanced cancer and can also play a role as a prognostic marker for cancer.
Targeting this pathway might help in the development of the therapy of many other symptoms, like neuromyelitis optica, uveitis and more recently, COVID-19 pneumonia.
However, since questions associated with IL-6 biology are still unanswered, the journey to understand the potential of treatment with the IL-6 pathway is not yet over. For instance, why are IL-6 levels high, and why does IL-6 signaling inhibition generate clinically significant advantages in patients with certain diseases like RA, but not all diseases that are linked with elevated IL-6 levels?
The answers to the above-discussed question will help researchers understand about how the IL-6 signaling pathway controls several autoimmune diseases and will aid in developing more exclusive therapy options for individual patients or patient subgroups.
A series of superior IL-6 and IL-6 receptor proteins has been developed by ACRO Biosystems to enable the development of IL-6 related drugs.
Image Credit: AcroBiosystems
Product features
- Availability of human and mouse species
- High purity: >95%
- High bioactivity verified by BLI, ELISA and cell-based assay
- Free protocols offered
- Various tags: Tag Free, His Tag, Avi & His Tag, Fc Tag
- Low endotoxin: <1.0 EU/μg
Product List. Source: AcroBiosystems
| Molecule |
Cat. No. |
Species |
Product Description |
Preorder/Order |
| IL-6 |
IL6-H8218 |
Human |
Biotinylated Human IL-6 Protein, epitope tag free, ultra sensitivity (primary amine labeling) |
Order |
| IL-6 |
IL6-H4218 |
Human |
ActiveMax® Human IL-6 Protein, Tag Free |
Order |
| IL-6 |
IL6-M5245 |
Mouse |
Mouse IL-6 Protein, His Tag |
Order |
| IL-6 |
IL6-M82Q7 |
Mouse |
Biotinylated Mouse IL-6 Protein, Avitag™,His Tag |
Preorder |
| IL-6 R alpha |
ILR-H4223 |
Human |
Human IL-6 R alpha / CD126 Protein, His Tag |
Order |
| IL-6 R alpha |
CD6-H82E8 |
Human |
Biotinylated Human IL-6 R alpha / CD126 Protein, Avitag™,His Tag |
Order |
| IL-6 R alpha |
ILR-H5259 |
Human |
Human IL-6 R alpha / CD126 Protein, Fc Tag |
Order |
| IL-6 R alpha |
ILR-M82E9 |
Mouse |
Biotinylated Mouse IL-6 R alpha / CD126 Protein, His,Avitag™ |
Order |
| gp130 |
ILT-M52H1 |
Mouse |
Mouse gp130 / CD130 / IL-6 R beta Protein, His Tag |
Order |
| gp130 |
ILT-M5252 |
Mouse |
Mouse gp130 / CD130 / IL-6 R beta Protein, Fc Tag |
Order |
Verification data
As confirmed by SDS-PAGE, high purity is above 95%.
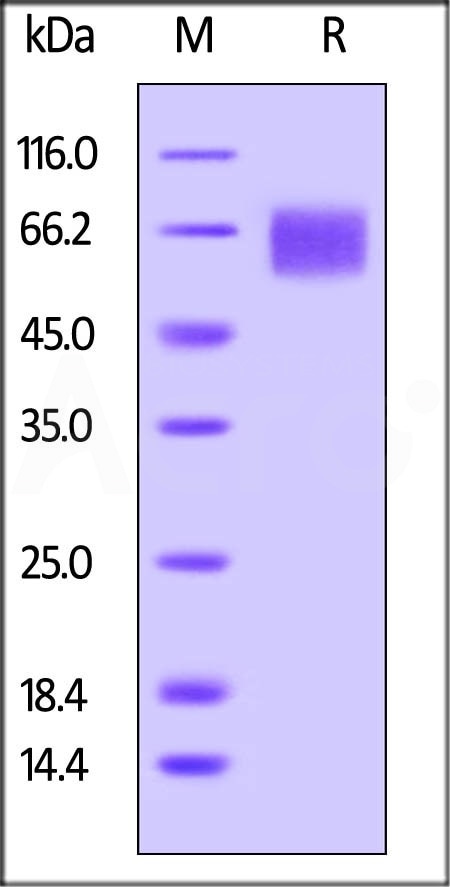
Biotinylated Human IL-6 R alpha, Avitag, His Tag (Cat. No. CD6-H82E8) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%. The protein has a calculated MW of 41.7 kDa and migrates as 55-70 kDa due to glycosylation. Image Credit: AcroBiosystems
High bioactivity verified by ELISA
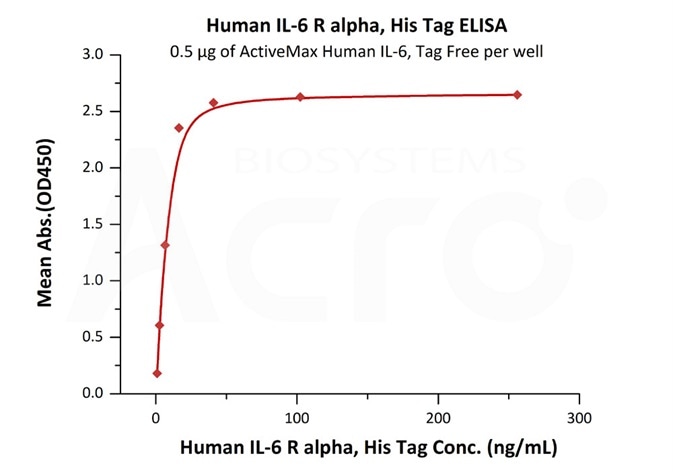
Immobilized ActiveMax® Human IL-6, Tag Free (Cat. No. IL6-H4218) at 5 μg/mL (100 μL/well) can bind Biotinylated Human IL-6 R alpha, Avitag, His Tag (Cat. No. CD6-H82E8) with a linear range of 4-125 ng/mL. Image Credit: AcroBiosystems
High bioactivity verified by BLI
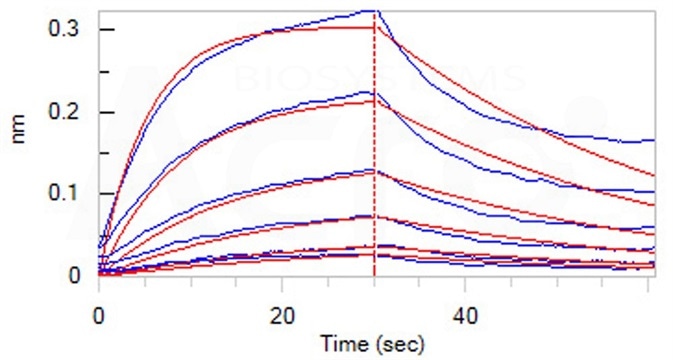
Loaded Human IL-6 R alpha, Fc Tag (Cat. No. ILR-H5259) on Protein A Biosensor, can bind ActiveMax® Human IL-6, Tag Free (Cat. No. IL6-H4218) with an affinity constant of 35.9 nM as determined in BLI assay (ForteBio Octet Red96e). Image Credit: AcroBiosystems
High bioactivity verified by cell-based assay
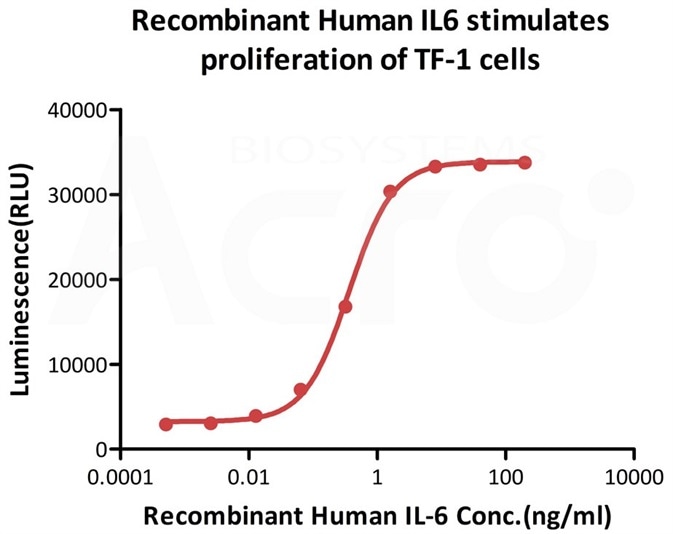
ActiveMax® Human IL-6, Tag Free (Cat. No. IL6-H4218) stimulates proliferation of TF-1 human erythroleukemic cell line. The EC50 for this effect is 0.2856-0.3636 ng/mL. Image Credit: AcroBiosystems.
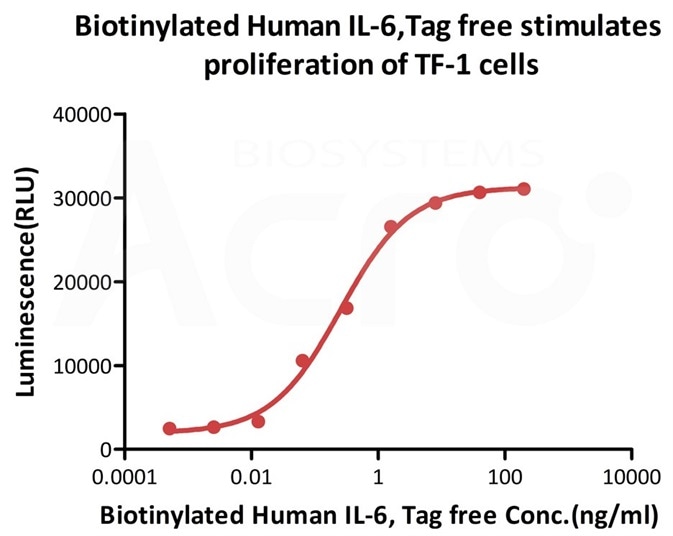
Biotinylated Human IL-6, epitope tag free, primary amine labeling (Cat. No. IL6-H8218) stimulates proliferation of TF-1 human erythroleukemic cell line. The EC50 for this effect is 0.2532-0.4489 ng/mL. Image Credit: AcroBiosystems.
References
- Translating IL-6 biology into effective treatments. Nat Rev Rheumatol 16, 335–345 (2020). Authors: Choy, E.H., De Benedetti, F., Takeuchi, T. et al.
- IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy?. Pharm Med 34, 223–231 (2020). Authors: Atal, S., Fatima, Z.
- Computer-aided prediction and design of IL-6 inducing peptides: IL-6 plays a crucial role in COVID-19. Briefings in Bioinformatics. 2021, 22(2): 936–945. Authors: Anjali Dhall et al.
- Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA. 2021,326(6):499–518. Authors: The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group.
- Why tocilizumab could be an effective treatment for severe COVID-19?. J Transl Med 18, 164 (2020). Authors: Fu, B., Xu, X. & Wei, H.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.